Question
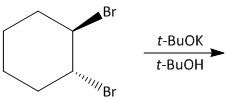
Question: Which is the final main product of the following reaction of trans-\[1,2\]-dibromocyclohexane? ![]...
Which is the final main product of the following reaction of trans-1,2-dibromocyclohexane?
A. 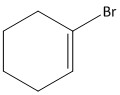
B. 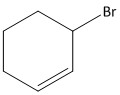
C. 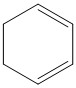
D. 
Solution
Cyclohexane exists in chair and boat conformations. The boat form is more stable conformer, but during a reaction it transforms into chair form. t−BuOK is a very strong base used for abstracting protons.
Complete step by step answer:
The trans-1,2-dibromocyclohexane exists in the form of chair and boat conformation. The boat conformation is of lower energy and more stable and the chair conformation is of higher energy and less stable due to diaxial interactions between the large bromine and hydrogen atoms.
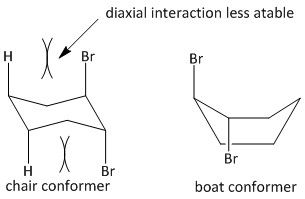
For the given reaction, when trans-1,2-dibromocyclohexane was treated with potassium t-butoxide in t-butanol, an elimination reaction will occur. Potassium t-butoxide is known as a very strong base and used for abstraction of protons.
For the given compound the proton present on the carbon adjacent to the carbon attached to bromine will be abstracted by the base. This will result in two E2 types of elimination reactions causing release of bromine atoms in the form of HBr.
For the E2 elimination an antiperiplanar orientation is required. Such orientation is only possible for a chair conformer and not a boat conformer. The hydrogen atoms at the C3 and C6 position of the Cyclohexane ring are antiperiplanar with the bromine atoms at C1 and C2.
The mechanism for the E2 elimination is as follows:
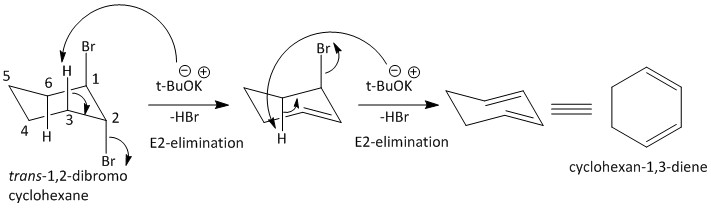
Thus the option C is the correct answer, i.e. Cyclohexane-1,3-diene.
Note:
Elimination reactions are of various types like E1, E2 and E1cb. Unlike E2 which is bimolecular with respect to substrate and the base, E1 is unimolecular which includes formation of stable carbocation and E1cb which is unimolecular conjugate base elimination. The stereochemistry of the product varies according to the type of elimination.
