Question
Question: Which is the correct statement about \(C{{r}_{2}}O_{7}^{2-}\) structure? A.It has neither \(Cr-Cr\...
Which is the correct statement about Cr2O72− structure?
A.It has neither Cr−Cr bonds nor O−O bonds.
B.It has one Cr−Cr bond and six O−O bonds.
C.It has no Cr−Cr bonds and six O−O bonds.
D.It has one Cr−Cr bond and seven Cr−O bonds.
Solution
We know that the Dichromate ion (Cr2O72−) is an anionic part of its salt. Dichromate ion is one of the divalent inorganic anions. These are oxyanions having +6 oxidation state of Chromium. These are very good oxidizing agents due to the attachment of oxygen atoms.
Complete answer:
As we know the concept of chemical bonding and structures of ions. Also, the bond length is defined as the equilibrium distance between the nuclei of two bonded atoms in a molecule. Bond lengths are expressed in terms of Angstrom or picometer. The two Cr−O bonds which share the single oxygen atom are longer than the six Cr−O terminal bonds that are equivalent. The salt of the dichromate ion is used for oxidation and reduction reactions. Dichromate poses an orange colour. The dichromate dianion consists of two tetrahedra sharing oxygen at the common corner.
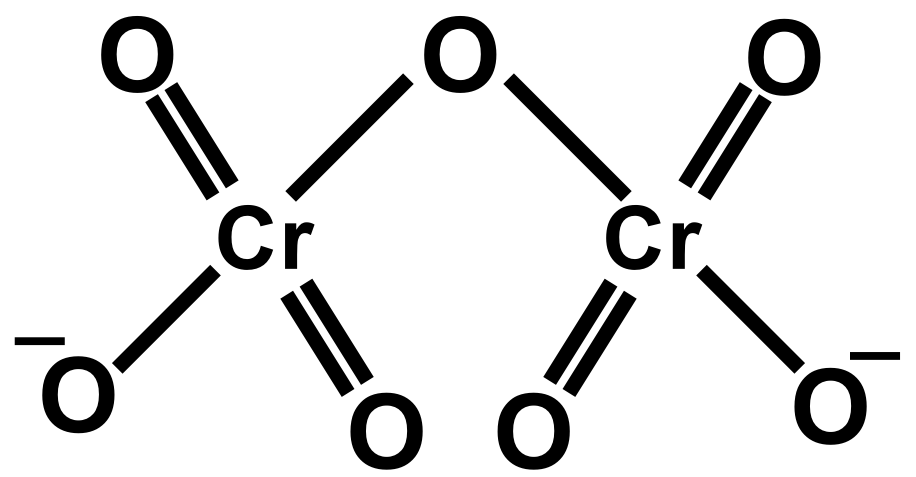
In dichromate dianion structure the negative charge can be on any oxygen attached to Cr thus all the Cr−O are equivalent and since one Cr−O−Cr bond. Dichromate is a divalent inorganic anion obtained by removal of both protons from dichromic acid. It is a chromium oxoanion and a divalent inorganic anion. It is a conjugate base of a hydrogen dichromate.
Therefore, the correct answer is option D.
Note:
Remember that the resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by a single Lewis formula. A molecule or ion with such delocalized electrons is represented by several resonance structures.
