Question
Question: Which is not true about \(Si{{O}_{2}}\)? (A) It is a network solid (B) It is attacked by molten ...
Which is not true about SiO2?
(A) It is a network solid
(B) It is attacked by molten NaOH
(C) It is attacked by HF
(D) It is the basic structural unit of silicates
Solution
To solve this question, we first need to know what is SiO2. Silicon dioxide (SiO2) or silica is a white amorphous chemical compound and an oxide of silicon. It is found in living organisms and exists in nature as quartz.
Complete answer:
Let us scrutinize the given statements
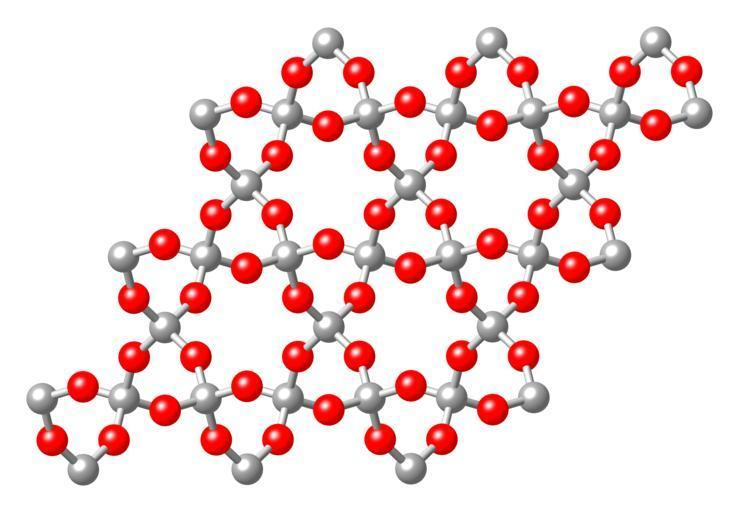
(A) The statement that silicon dioxide (SiO2) is a network solid is true.
When the atoms are bonded by covalent bonds in a chemical compound in a continuous network that extends throughout the material is known as a network solid or an atomic crystalline solid.
Silicon dioxide (SiO2) has a three-dimensional continuous network of SiO2 units and hence it is a network solid.
(B) The statement that silicon dioxide (SiO2) is attacked by molten NaOH is true.
When hot and molten (i.e., concentrated) sodium hydroxide (NaOH) reacts with silicon dioxide (SiO2) at a temperature of 900-1000∘C, sodium silicate (Na2SiO3) is formed along with water molecule. The reaction proceeds as follows:
SiO2+2NaOH900−1000∘CNa2SiO3+H2O
(C) The statement that silicon dioxide (SiO2) is attacked by HF is true.
When hydrogen fluoride (HF) attacks silicon dioxide (SiO2), hexafluorosilicic acid (H2SiF6 ) is produced along with the formation of water molecules. The reaction proceeds as follows:
SiO2+6HF→H2SiF6+2H2O
(D) The statement that silicon dioxide (SiO2) is the basic structural unit of silicates is false.
The basic structural units of silicates are SiO44−.
So, the answer to the question is option (D).
Note:
It should be noted that
- Silicon can be produced by reducing silicon dioxide (SiO2) with carbon.
- Halogen gases except fluorine do not essentially react with silicon dioxide (SiO2).
- Silicon dioxide (SiO2) does not react with most acids.
- Silicon dioxide (SiO2) is insoluble in water but dissolves in hydrofluoric acid (HF).
