Question
Question: Which is not the resonance structure of aniline? 
A. 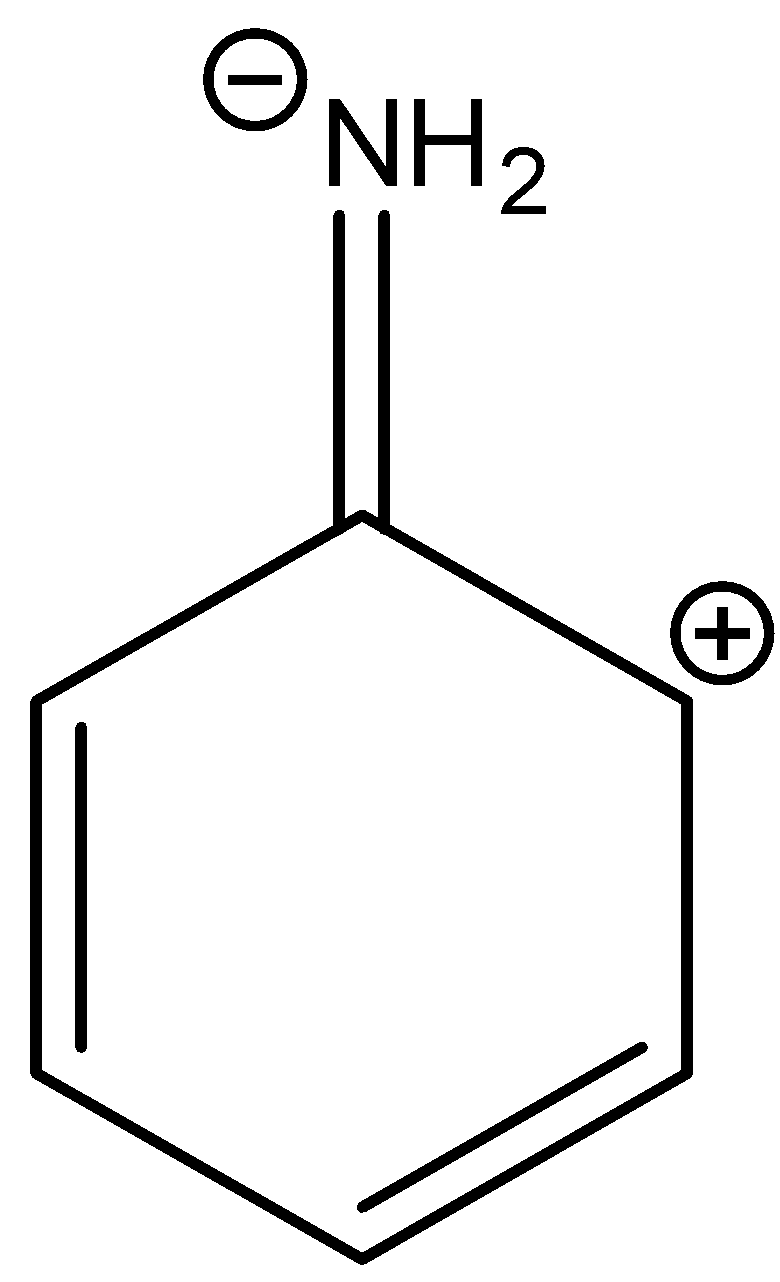
B. 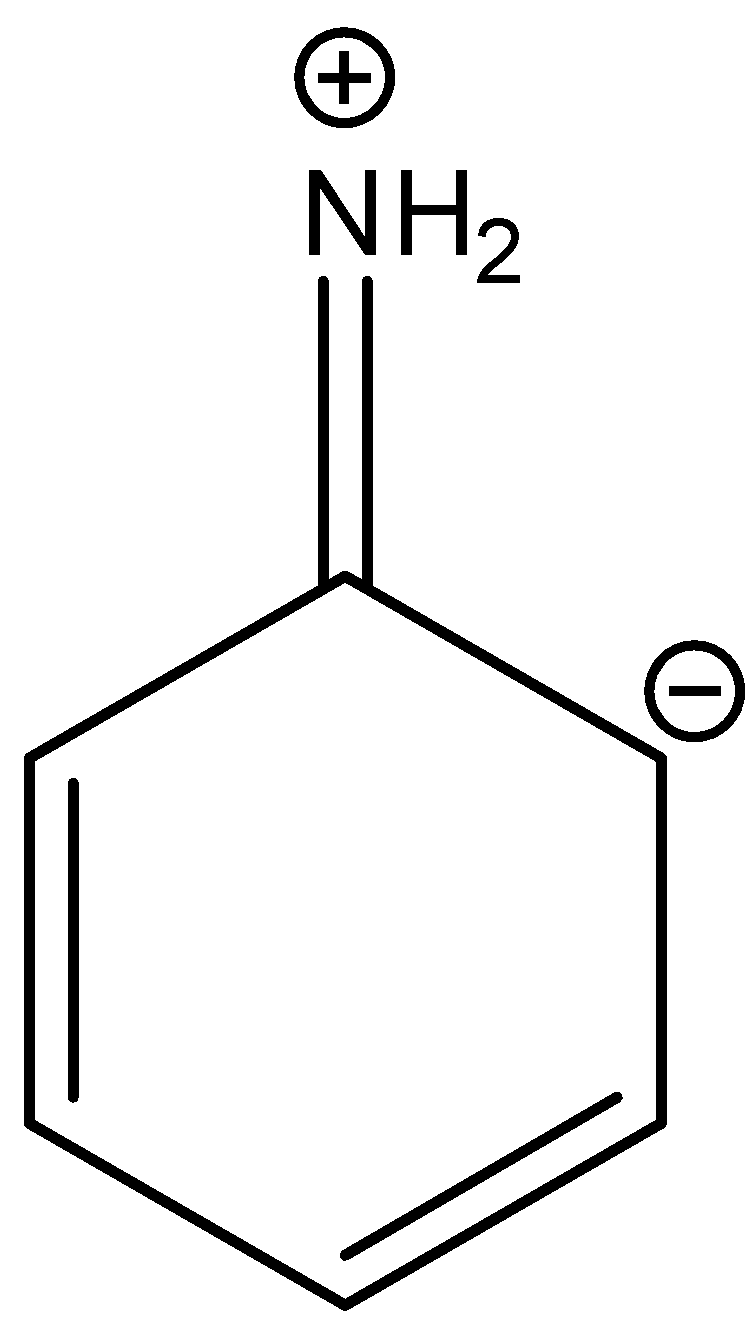
C. 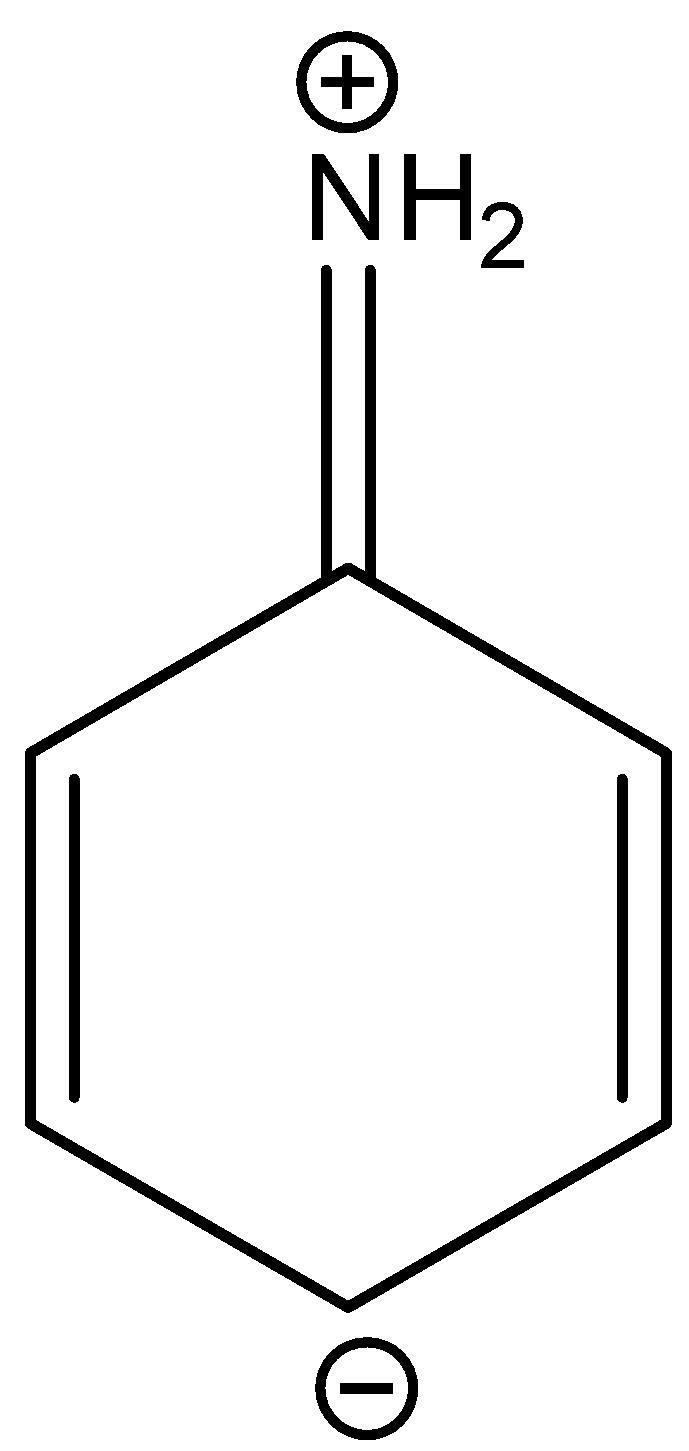
D. 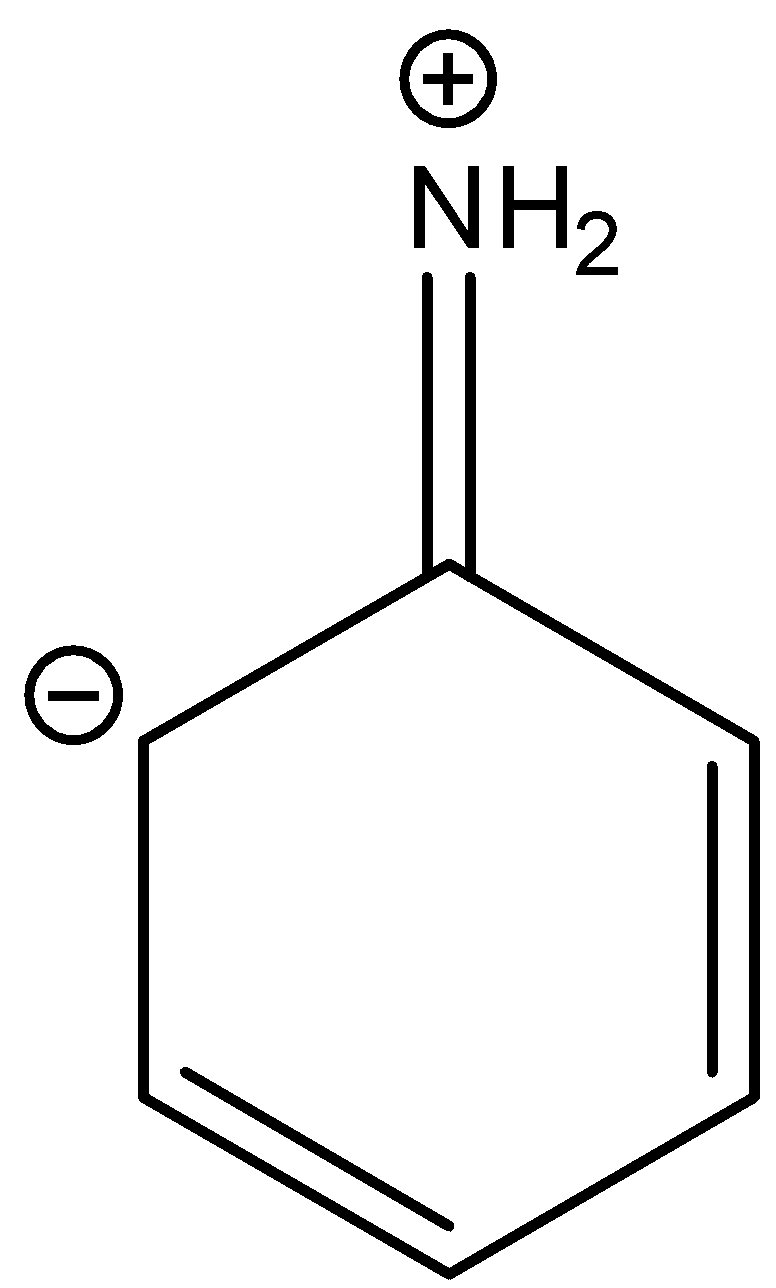
Solution
Aniline is an organic compound that has an aromatic ring in its structure. Aniline consists of a phenyl ring attached to an amine group. The structure of aniline is as follows. Due to the presence of lone pairs of electrons on nitrogen in amine groups of aniline undergoes resonance with the aromatic ring.
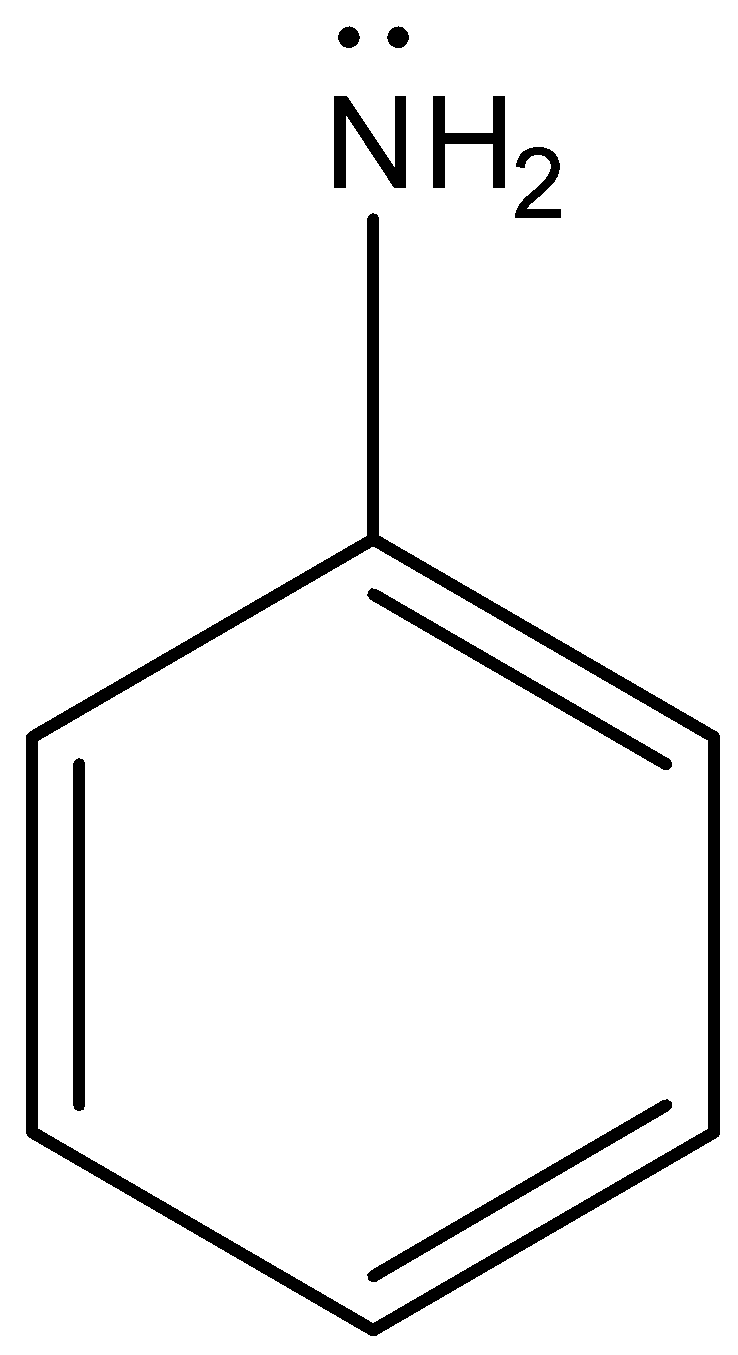
Complete step by step answer:
Aniline shows resonance structures due to the presence of a lone pair of electrons present in the anime group which is attached to a benzene ring.
The resonance structure of the aniline is as follows.
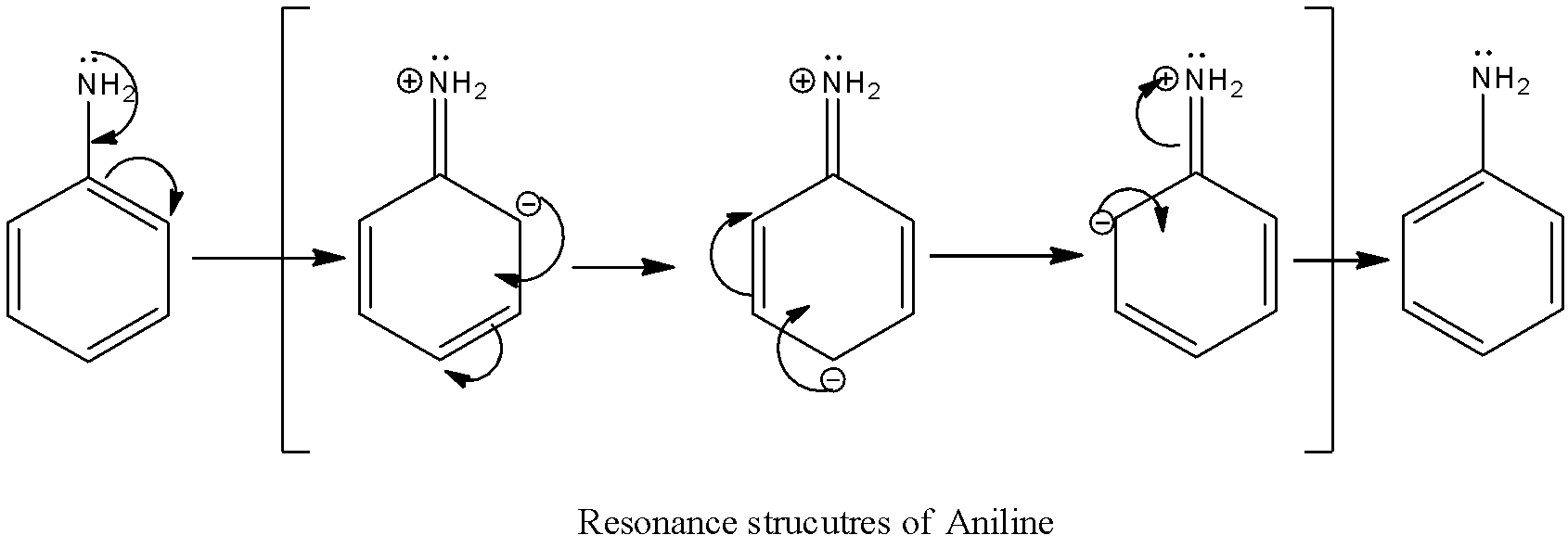
There are three resonance structures for aniline.
The resonance structures of aniline are due to the involvement of lone pairs of electrons on nitrogen.
In three resonance structures of aniline, the Nitrogen atom in amine group has a positive charge and one of the Carbon atoms in the ring (ortho or para to amine group in aniline) contains a negative charge.
There is no resonance structure containing a negative charge on nitrogen in amine groups. But in option A there is a negative charge on the nitrogen atom in the amine group.
So, the correct answer is “Option A”.
Note: In the resonance structures of aniline the negative charge is present at ortho and para positions to the amine group. Because of the presence of negative charge in aniline, electrophiles are going to react with aniline easily at ortho and para positions. So, amine in aniline directs electrophiles ortho or para positions. Therefore the amine group is called ortho or para directing group.
