Question
Question: Which is more stable, cis -\(1\)-ethyl-\(2\) -methylcyclohexane or trans -\(1\)-ethyl-\(2\)-methylcy...
Which is more stable, cis -1-ethyl-2 -methylcyclohexane or trans -1-ethyl-2-methylcyclohexane?
Solution
There is unfastened rotation approximately the carbon-to-carbon unmarried bonds (C–C) in alkanes. In contrast, the shape of alkenes calls for that the carbon atoms of a double bond and the 2 atoms bonded to every carbon atom all lie in an unmarried plane, and that every doubly bonded carbon atom lies withinside the middle of a triangle. This part of the molecule’s shape is rigid; rotation of approximately doubly bonded carbon atoms isn't feasible without rupturing the bond.
Complete Answer:
Trans -1-ethyl-2-methylcyclohexane will be more stable than cis-1-ethyl-2-methylcyclohexane
I'll examine the 2maximum solid chair conformations for every molecule. If you need to look at why those chairs are the maximum solid, see
Now, this is the maximum solid chair conformer for trans-1-ethyl-2-methylcyclohexane
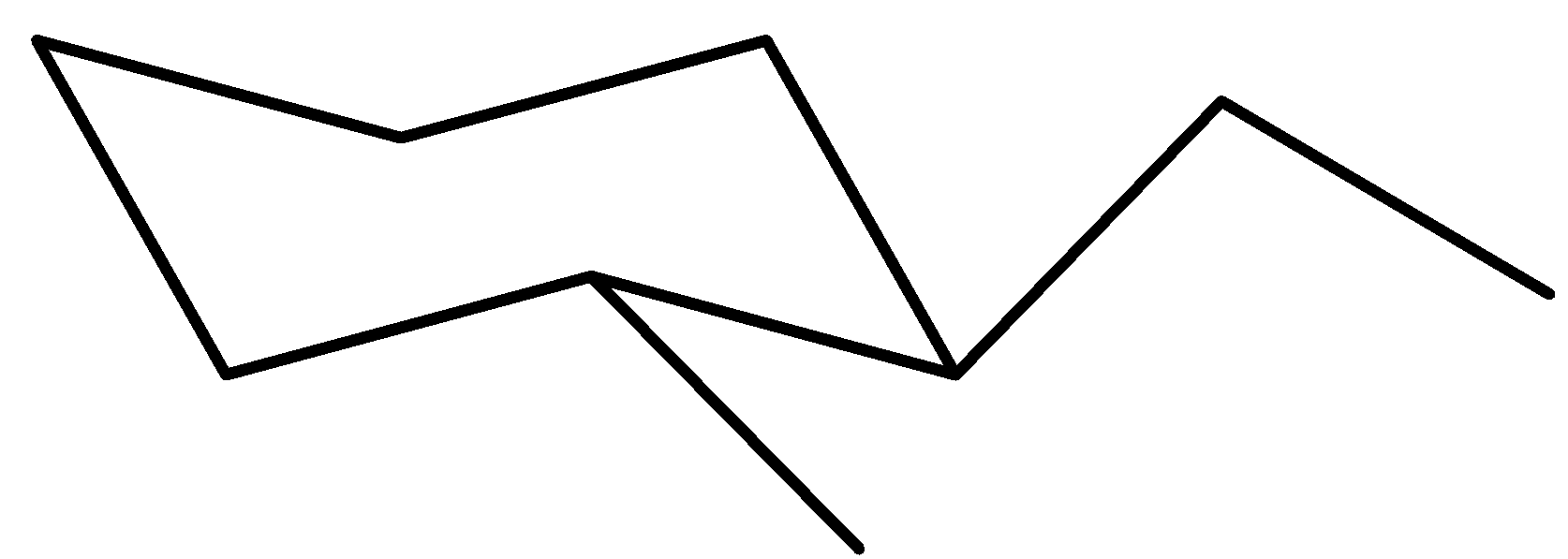
Notice which you have the ethyl organization connected to carbon (1) in UP function on an equatorial bond. The methyl organization is hooked up to carbon (2) in DOWN function on an equatorial bond as well.
Now examine the maximum solid chair for cis-1-ethyl-2-methylcyclohexane
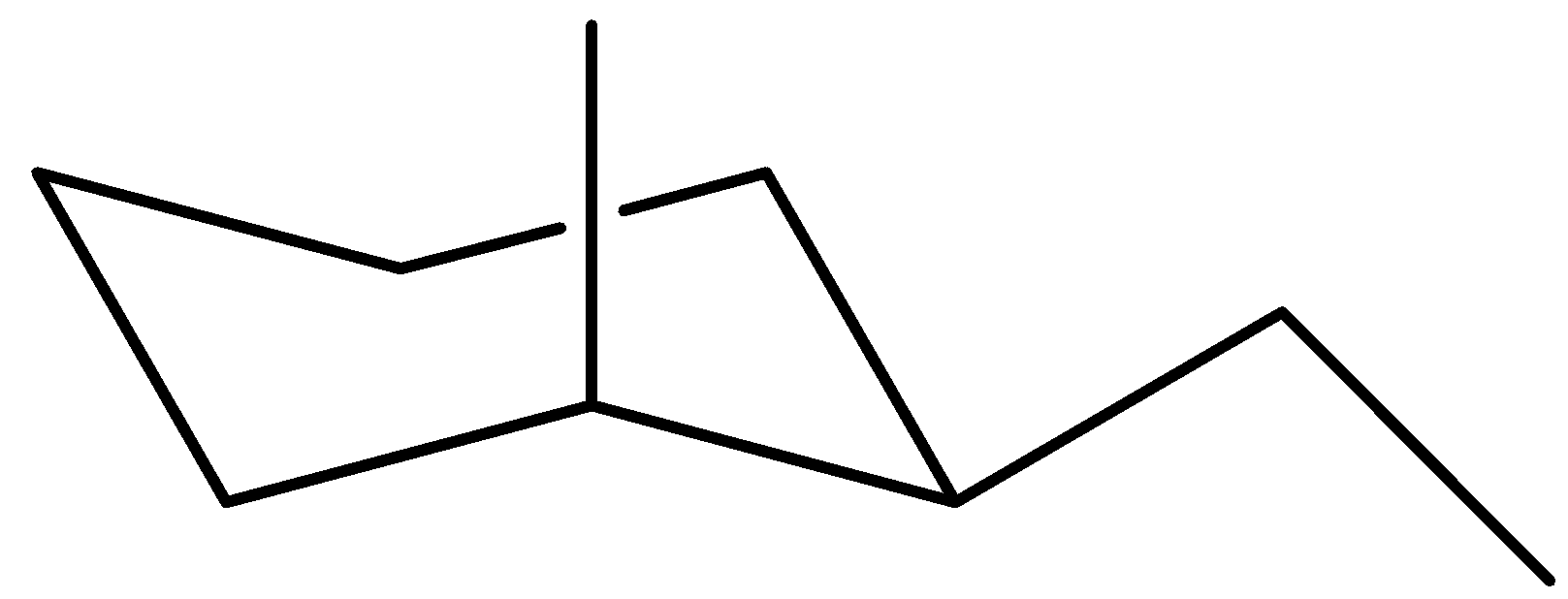
Once again, the ethyl organization is in UP function on carbon (1), however this time the methyl organization is in UP function on an axial bond. The reality that the methyl organization is on an axial bond will in the end decide which of those chair conformers are extra solid.
Ideally, extra solid chairs will have the bigger companies on equatorial bonds. As you may see, that's what takes place withinside the first chair. For the second chair, the methyl organization's function on an axial bond will purpose steric strain, as a way to lessen the steadiness of the chair.
As a result, trans-1-ethyl-2-methylcyclohexane has an extra solid chair conformer than cis-1-ethyl-2-methylcyclohexane.
Note:
If you may choose up both molecule from the web page and turn it over from pinnacle to bottom, you'll see that the 2 formulation are identical. Thus there are necessities for cis-trans isomerism:
1. Rotation needs to be restrained withinside the molecule.
2. There need to be no identical companies on every doubly bonded carbon atom.
