Question
Question: Which is called chromic anhydride? A) \(\text{ CrO }\) B) \(\text{ C}{{\text{r}}_{\text{2}}}{{\...
Which is called chromic anhydride?
A) CrO
B) Cr2O3
C) CrO3
D) CrO2
Solution
Anhydrides are the compounds which are obtained by the elimination of water molecules from the compound. The chromic anhydride is obtained by the removal of water molecules from the chromic acid. The structure of the chromic acid is a H2CrO4 .
Complete step by step answer:
Chromium (VI) oxide is also known as chromium trioxide. It is an inorganic compound and has a formula as CrO3 . The chromium trioxide is a powerful oxidizing agent. The structure of the chromium trioxide is as shown below,
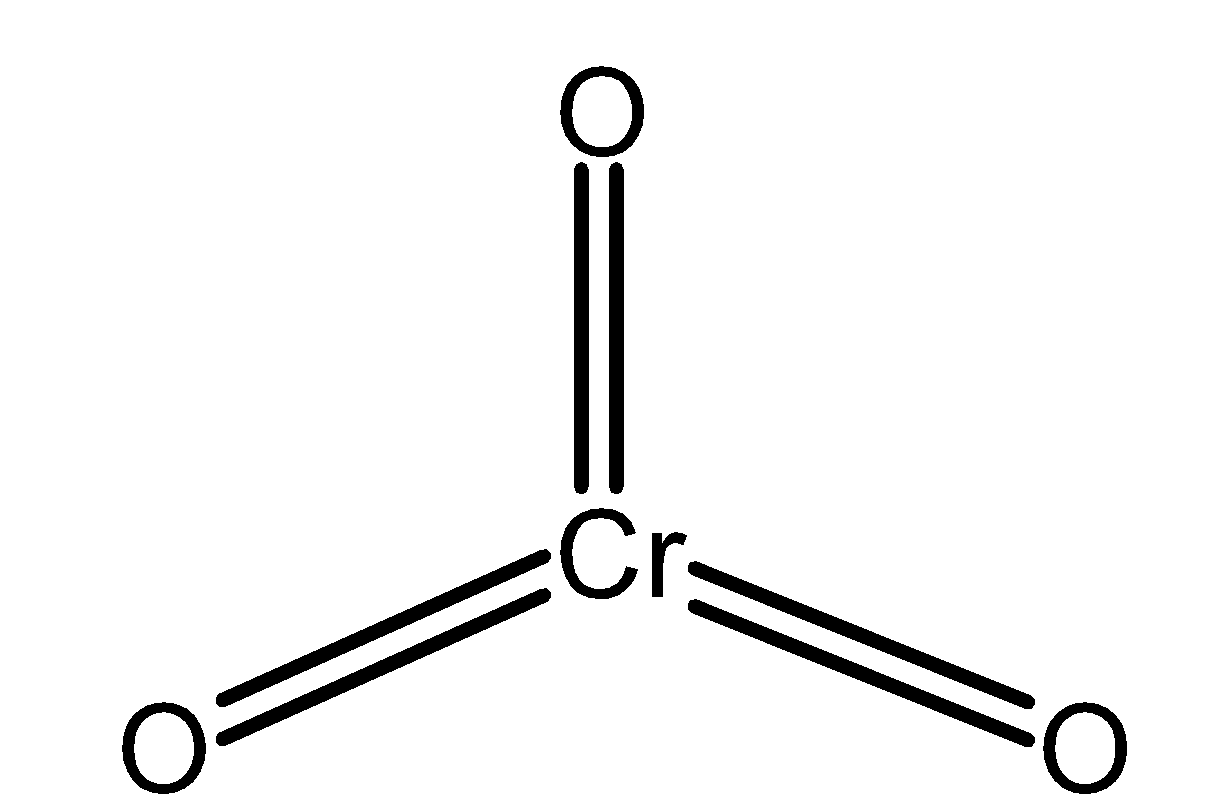
Chromium trioxide is an acidic anhydride of chromic acid. Chromium trioxide is generated by the treatment of sodium chromate with sulphuric acid. The reaction of preparation of chromium trioxide is as follows,
H2SO4 + Na2Cr2O7 → 2CrO3 + Na2SO4 + H2O
Chromium trioxide is carcinogenic, toxic, and highly corrosive. It is a strong oxidizer and ignites when organic compounds are in contact. The chromium trioxide is an anhydride of chromic acid. The anhydrides are the compounds which are obtained after removing the water molecules from the particular compound. The chromic acid has the formula as, H2CrO4 . When the water molecules is removed from the chromic we get,
H2CrO4 → CrO3 + H2O
Therefore, the chromium (VI) trioxide is known as the chromium anhydride. Thus, the structure of chromic anhydride is CrO3 .
Hence, (C) is the correct option.
Additional information:
Uses of chromium trioxide are as follows,
-It is used as the oxidizing agent in chrome plating
-It is used in the production of synthetic ruby
-It is used in the aerospace applications
Note: The anhydride which is capable of forming an acid in the solution is known as the acidic anhydride. Chromic anhydride is an acidic anhydride as it generates a chromic acid in the solution. There are basic anhydride too, for example, sodium oxide Na2O .
