Question
Question: Which is/are correct representations of boranes? A. \({B_n}{H_{n + 4}}\) B. \({B_n}{H_{n + 6}}\...
Which is/are correct representations of boranes?
A. BnHn+4
B. BnHn+6
C. BnH2n+2
D.None of these
Solution
Boranes is basically the name given to the class of synthetic hydrides of boron. These were often labelled as electron deficient because of their multicenter bonding. Usually they have a generic formula BXHY .
Complete step by step answer:
Basically, the hydrides of boron are known as boranes. They are further classified as closo, nido, hypo and conjunto boranes. The key difference between closo, and nido boranes is that in closo structure, it has n+1 skeletal bonding electron pairs whereas in case of nido structure there is n+2 skeletal bonding electron pairs. They are non-classically bonded compounds i.e. there are not enough electrons to form two center, two electron bonds between all pairs of adjacent atoms in the molecule. The boron hydrides were first systematically synthesized and characterized during the period 1912 to 1973 by the German chemist Alfred Stock.
Now, the boranes that were prepared by stock had the general formula BnHn+4 and BnHn+6 . The hydrides of boron are more numerous than those of any other element except carbon. The simplest isolable borane is diborane i.e. B2H6 . It is one of the most extensively studied and most synthetically useful chemical intermediates. This substance is highly unstable at room temperature with a sweet odor. The structures of borane and diborane are as shown:
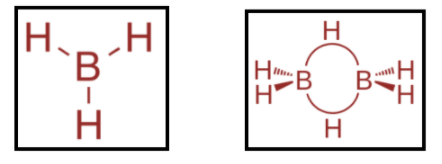
Hence, option A and option B, both are correct.
Note: Boranes have been widely studied as potential fuel for rockets and for automotive uses. They further act as ligands in the coordination compounds. They also find their applications in medicine, materials and thin films.
