Question
Question: Which has the maximum dipole moment? (A)  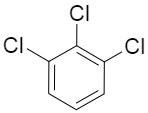
(B) 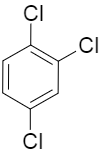
(C) 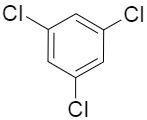
(D) 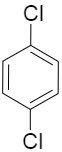
Solution
Hint : As we know that dipole moment is basically a quantity which tells us about the two opposites charged which are separated by a distance thereby determining the size of the partial charges on that molecule and its polarity. Dipole moment is generally added when charges are similar and cancelled when charges are different.
Complete Step By Step Answer:
We need to understand that the dipole moments of the structure having the same charges in exactly opposite directions, would nullify each other and hence the dipole moment would become zero.
If we consider the options given in the question, the options C and D have the chlorines attached to 1,3,5 positions in option C and the para positions to each other in option D. In these two cases the dipole moment would be zero as they cancel out the effect of each other.
Now if we consider option B, we can see that two of the chlorine substituents are present at the para position to each other and so they are cancelling out each other’s effect. And only one chlorine substituent is contributing to the dipole moment of the whole molecule.
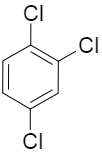
If we consider the option A, the dipole moment of all the three chlorine substituent is contributing to the net dipole moment of the molecule as none of them are getting nullified by the presence of others.
So, the correct option would be option A.
Note :
The polarity of any molecule between the atoms, results in the formation of partial charge between them. The polar nature could be measured by the use of dipole moment. Always remember that Dipole moment is a vector quantity and therefore, it can be zero because the two opposite charges cancel out each other and it can be positive because two similar charges can add up the bond dipoles.
