Question
Question: Which has the largest \( O - N - O \) bond angle? (A) \( N{O_2} \) (B) \( NO_2^ + \) (C) \( ...
Which has the largest O−N−O bond angle?
(A) NO2
(B) NO2+
(C) NO2−
(D) NO3−
Solution
The bond angle is the mean angle between the orbitals which have bonding electron pairs; those are surrounding the central atom in a molecule. Each different compound has different angles and for the formation of bond angles, there should be at least three atoms and two bonds.
Complete answer:
We know that bond angle is the angle which is found between two covalent bonds which develop from the same atom. There are some factors in which bond angle depends which are:
Electronegativity and the lone pair are one factor which affect bond angle. When we decrease the electronegativity and increase the lone pair then the bond angle decreases.
Forces of attraction or repulsion between atoms of molecules are another factor which affects bond angle. It may increase or decrease the bond angle.
Hybridization is also the factor which affects the bond angle. When the s character of hybrid orbital increases then bond angle increases.
The concept of combining atomic orbitals to generate new hybrid orbitals which are suitable for the pairing of electrons to form chemical bonds is known as hybridization. There are two bond pairs and one lone pair in NO2 , thus it has sp2 hybridization and has triangular planar geometry. So, its bond angle is 134∘ . In NO2+ there are two bond pairs but zero lone pairs so hybridization of NO2+ is sp and its geometry is linear. Its bond angle is 180∘ . Now in NO2− there are two bond pairs and one lone pair with negative charge. Because of negative charge, there would be repulsions between negative charge and lone pair which decreases the bond angle. So the bond angle of NO2− will be less than NO2 . Thus the bond angle of NO2− is 115∘ . There are four bond pairs and zero lone pairs in NO3− , thus it has sp2 hybridization and has trigonal planar geometry. The bond angle of NO3− is 120∘ .
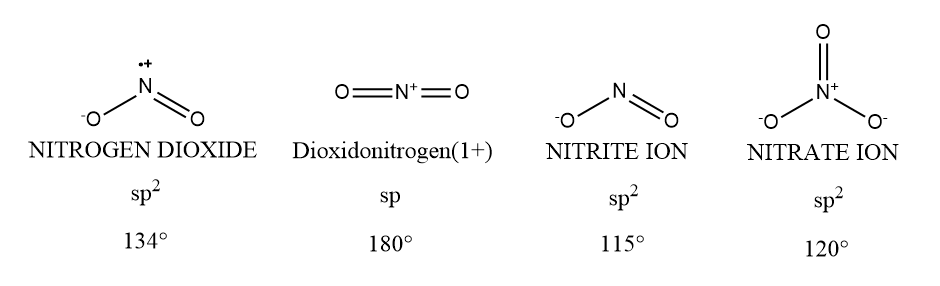
Here we can see the bond angle of NO2+ is the largest among the other compounds. Its bond angle is 180∘ .
So, option (b) is correct.
Note:
In the given question we use hybridization over other factors as the atoms in all molecules are the same but their charges are different. Lone pair-lone pair repulsions are stronger than lone pair-bond pair repulsions and bond pair- bond pair repulsions.
