Question
Question: Which group can be represented by the Lewis dot structure shown in the figure? 
A. Group IA
B. Group IIA
C. Group IIIA
D. Group VIA
E. Group VIIA
Solution
We have to know that the Lewis electron dot charts use dabs to address valence electrons around a nuclear image. Lewis electron spot outlines for particles have less (for cations) or more (for anions) specks than the comparing atom.
Complete answer:
We have to know that the Lewis dot structure represents the sharing of electrons between particles in covalent or polar covalent bonds. The spots in a Lewis speck structure address an atom's valence electrons and the situation of the dabs show how the electrons are circulated in a particle.
The rules for drawing Lewis dot structure has to be given below,
Decide the complete number of valence electrons.
Compose the skeleton design of the atom.
Utilize two valence electrons to frame each bond in the skeleton structure.
Attempt to fulfill the octets of the iotas by circulating the excess valence electrons as nonbonding electrons.
The given Lewis dot structure is drawn below,
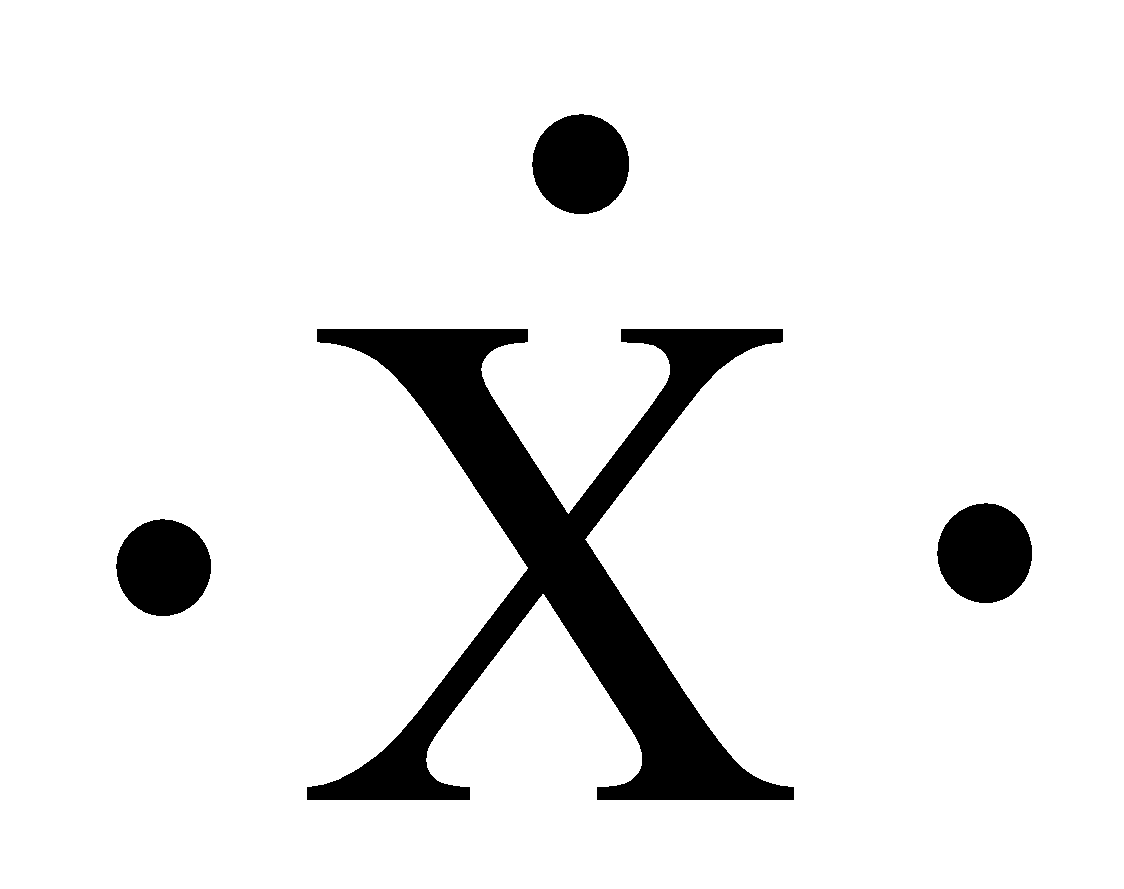
The number of valence electrons present in the above Lewis structure is three.
Therefore, three valence electrons are present in the group IIIA.
This group IIIA of the occasional table incorporates the metalloid boron, just as the metals aluminum, gallium, and indium. Boron frames generally covalent bonds, while different components in group IIIA structure for the most part ionic bonds.
Therefore, the correct option is (C).
Note:
We have to know that, the metallic character increments down the gathering. All are metals with the exception of boron, which is a nonmetal. Gallium has a strangely low softening point and a significant huge fluid reach. Both gallium and indium are utilized to make semiconductors.
