Question
Question: Which group can be represented by the Lewis dot structure shown in the figure? 
(a)- Group I A
(b)- Group II A
(c)- Group III A
(d)- Group VI A
(e)- Group VII A
Solution
The naming of the group in the given options was given by Mendeleev in his Mendeleev's periodic table. If the element has three dots around the element means the element has three valence electrons so, it will belong to group 13.
Complete answer:
Lewis dot structure is used to tell the number of valence electrons in the element. And it is represented by putting the dots equal to the number of valence electrons. For an element, write its symbol and put the number of dots around the symbol.
The naming of the group in the given options was given by Mendeleev in his Mendeleev's periodic table. The given options are a group I A is known as group 1, group II A is known as group 2, group III A is known as group 13, group VI A is known as group 16, and group VII A is known as group 17.
The given figure in the question is:
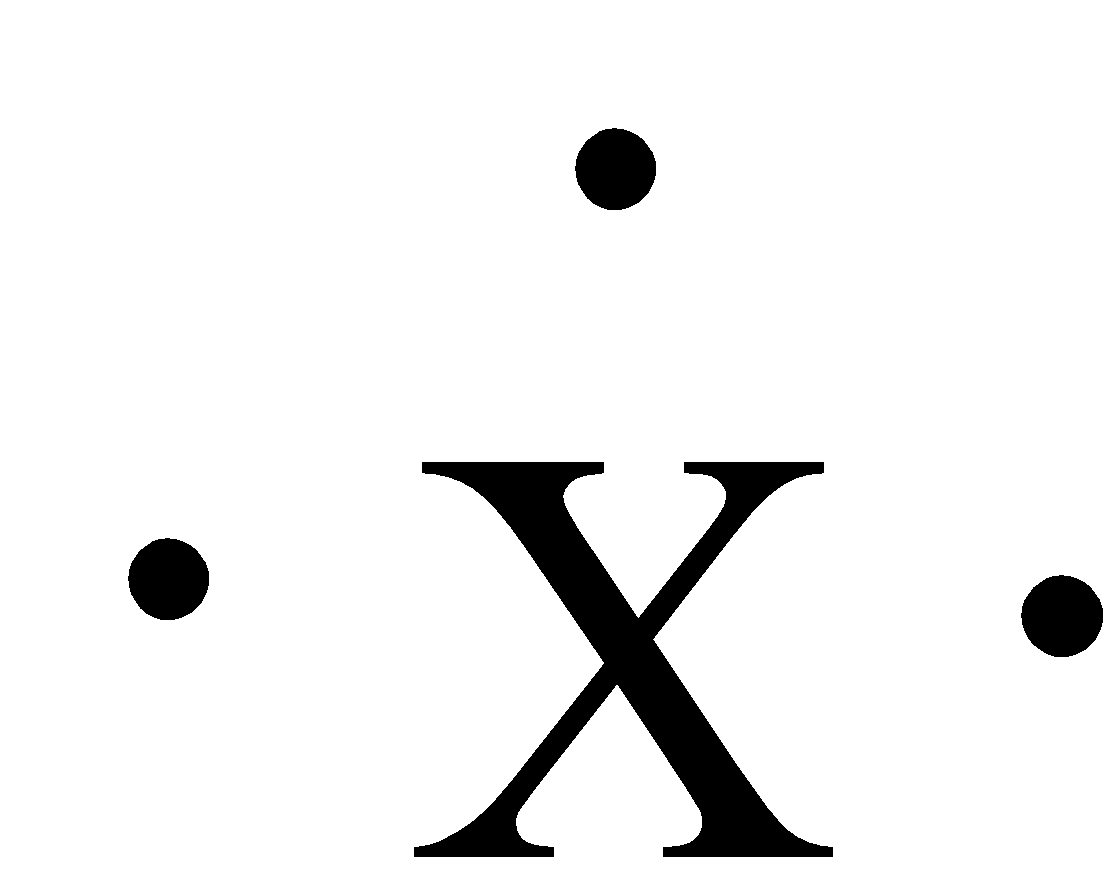
The numbers of dots around the symbol are three. Group 13 has 3 valence electrons, 2 from the s-orbital and 1 from the p-orbital. The elements that have this Lewis dot structure are boron, aluminium, gallium, indium, and thallium. So, this Lewis dot structure is for Group III A.
Therefore, the correct answer is an option (c)- Group III A.
Note:
Valence electrons are present in the valence shell and the valence shell is decided by the shell number so, all the subshells in the shell will be considered. For example, if the last shell is 2 and electrons are in 2s and 2p, then both of them will be considered for valence electrons.
