Question
Question: Which gives only glucose by hydrolysis? A. Sucrose B. Raffinose C. Maltose D. Galactose...
Which gives only glucose by hydrolysis?
A. Sucrose
B. Raffinose
C. Maltose
D. Galactose
Solution
In hydrolysis a molecule of water is added to the chemical compound. This causes both the compound and water to split up. In such a reaction the parent product gains a hydrogen ion. Hydrolysis reaction is the reverse of condensation reaction in which two compounds join together leaving behind a water molecule.
Complete step by step answer: In this question we have to find the compound which yields only glucose on hydrolysis. Hydrolysis is a reaction in which a reactant is made to split into two or more products with the addition of water molecules. In this reaction the parent product gets hydrogen ions.
Glucose is a type of sugar. Its chemical formula is C6H12O6.
Sucrose is a disaccharide means it is composed of two monosaccharide. Monosaccharide is simple sugars. These are the simplest unit of carbohydrates. These can’t be hydrolyzed further. Chemical formula of sucrose is C12H22O11. When hydrolyzed sucrose gives two products that is: glucose and fructose.
C12H22O11+H2OHClC6H12O6+C6H12O6 Sucrose Glucose Fructose
Chemical formula of glucose and fructose is the same but the difference lies in their structure.
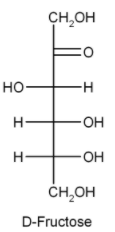
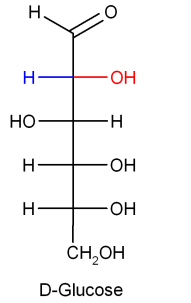
This is the simple difference in the structure of glucose and fructose. As we can see sucrose on hydrolysis gives two products but we need only one that is glucose. This means sucrose is not the answer.
Raffinose is a trisaccharide. Means it is made up of three monosaccharides. Chemical formula of raffinose is C18H32O16 . Raffinose on hydrolysis yields galactose, glucose and fructose.
C18H32O16+H2OC6H12O6+C6H12O6+C6H12O6 Raffinose Glucose Fructose Galactose
We need only glucose as the product but three products are coming from hydrolysis of Raffinose. This means raffinose is also not the answer.
Maltose is a disaccharide. Chemical formula of maltose is C12H22O11 . On hydrolysis of maltose we get two molecules of glucose.
C12H22O11+H2OHCl2C6H12O6 Maltose Glucose
So, this is our required product that is glucose. We needed a compound which gives only glucose as a product.
So, the correct answer is “Option C”.
Note: Monosaccharides are the simplest unit of carbohydrates. These can’t be hydrolyzed further. Disaccharides are made up of two monosaccharides. This means on hydrolysis of disaccharides we get two monosaccharides. Similarly there exist trisaccharides and polysaccharides as well.
