Question
Question: Which gas will come out when a mixture of methane, ethylene, and acetylene is passed through a Wolf ...
Which gas will come out when a mixture of methane, ethylene, and acetylene is passed through a Wolf bottle containing ammoniacal cuprous chloride?
A. methane
B. acetylene
C. a mixture of methane and ethylene
D. organic mixture
Solution
Ammoniacal cuprous chloride has a molecular formula 2[Cu(NH3)2]Cl . It is an organic compound that reacts with terminal alkynes. This solution is used to test the presence of a triple bond in an organic compound. Ammoniacal cuprous chloride reacts with terminal alkynes to form a red precipitate of copper salt.
Complete Step by step answer: Ammoniacal cuprous chloride is a solution of cuprous chloride in ammonia. It is prepared by mixing cuprous chloride in water and ammonia till we obtain a blue-colored solution. This solution is used to test the presence of a triple bond in an organic compound. Ammoniacal cuprous chloride reacts with terminal alkynes to form a red precipitate of copper salt.
Now we are given a mixture of methane, ethylene, and acetylene. Let us see what happens when this mixture is passed through ammoniacal cuprous chloride solution
Methane is an organic compound with a molecular formula CH4. It is a form of gas and is the simplest hydrocarbon. When it is passed through ammoniacal cuprous chloride, no reaction will occur. Because there is no triple bond present
CH4+2[Cu(NH3)2]Cl→No reaction
Similarly, ethylene has a molecular formula C2H4 . It has a structure 2HC=CH2 . When it is passed through ammoniacal cuprous chloride, no reaction will occur. Because there is no triple bond present
C2H2+2[Cu(NH3)2]Cl→No reaction
Acetylene has a molecular formula C2H2 and structure . Since it has a triple bond it will undergo a reaction with ammoniacal cuprous chloride.
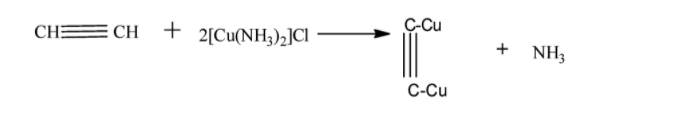
Thus the correct option is C because methane and ethylene are forms of gases and no reaction is occurring with ammoniacal cuprous chloride.
Note: It is important to know what are terminal alkynes. Terminal alkynes are those alkynes in which at least one H atom is bonded to a triple bond carbon atom. Similarly, we have terminal alkenes in which a double bond is at the end of the carbon chain.
