Question
Question: Which functional group is introduced in phenol when it reacts with chloroform and dilute sodium hydr...
Which functional group is introduced in phenol when it reacts with chloroform and dilute sodium hydroxide?
A. −CHCl2
B. −CHO
C. −CH2Cl
D. −COOH
Solution
When phenol that is C6H6O reacts with chloroform that is and sodium hydroxide that is NaOH, it is called Reimer–Tiemann reaction. This reaction introduces the aldehyde group in phenol CHCl3 at ortho position. At ortho position, intramolecular H bonding occurs.
Complete answer:
The Reimer–Tiemann reaction is a very famous reaction which is used to convert phenol C6H6O into salicylaldehyde C7H6O2 . It was discovered by Karl Reimer and Ferdinand Tiemann. This reaction has two steps .In first step phenol reacts with chloroform CHCl3 and NaOH to produce an intermediate .In the second step this intermediate undergoes hydrolysis to give 2-hydroxy benzaldehyde.
It is important we understand the mechanism of the reaction . In the first step of the mechanism OH− of NaOH reacts with Hof CHCl3 to form water and trichloromethyl anion. It is called deprotonation. Similarly deprotonation of phenol occurs.
CHCl3+OH−→H2O+CCl3−
Since chlorine is a group 17 element and highly electronegative, it is a very good leaving group .So therefore chlorine leaves trichloromethyl anion and negative charge on trichloromethyl anion is neutralized. The product formed in the second step reacts with deprotonated phenol to form an intermediate product. This further undergoes hydrolysis to give salicylaldehyde. The product formed in the second step reacts with deprotonated phenol to form an intermediate product. This further undergoes hydrolysis to give salicylaldehyde.
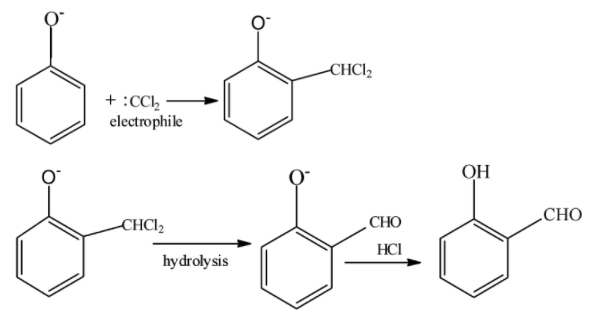
**So the correct option is B
Note:**
It is important to note that instead of NaOH we can also use KOH. NaOH or KOH helps in deprotonation of chloroform and phenol. So it becomes necessary to use a strong base to begin the reaction.
