Question
Question: Which does not have a carboxyl group? A. Picric acid B. Ethanoic acid C. Salicylic acid D. B...
Which does not have a carboxyl group?
A. Picric acid
B. Ethanoic acid
C. Salicylic acid
D. Benzoic acid
Solution
We know that the functional group is the atom or group of atoms that decides the chemical reactivity of the compound. Some examples of functional groups are aldehyde (-CHO), alcohol (-OH), ketone (R-CO-R), carboxyl group etc. The structure of the carboxyl group is R –COOH.
Complete step by step answer:
Let’s understand the structure of the carboxyl group. In the carboxyl group, a carbon is singly bonded to an alcohol group and doubly bonded to an oxygen atom. The structure of the carboxyl group is,
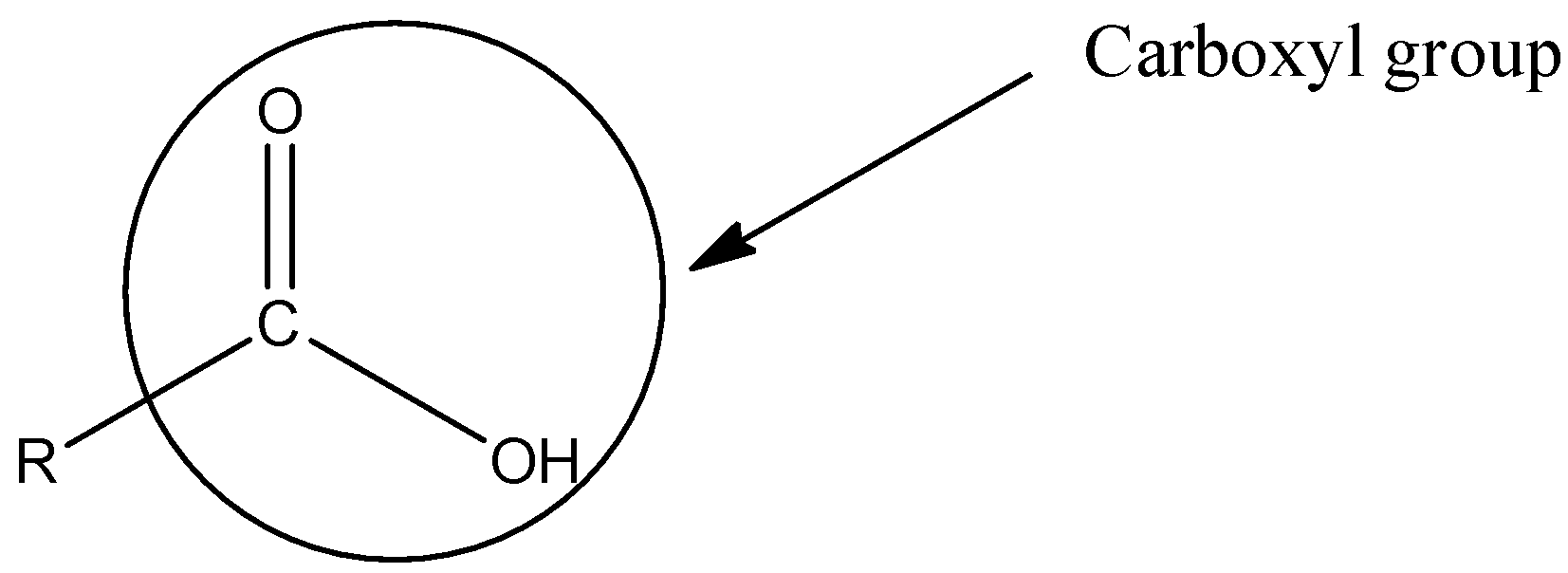
Here, R represents any alkyl or aryl group.
Now, come to the question. We have to identify the molecule which does not have a carboxyl group.
Option A is picric acid. The structure of picric acid is,
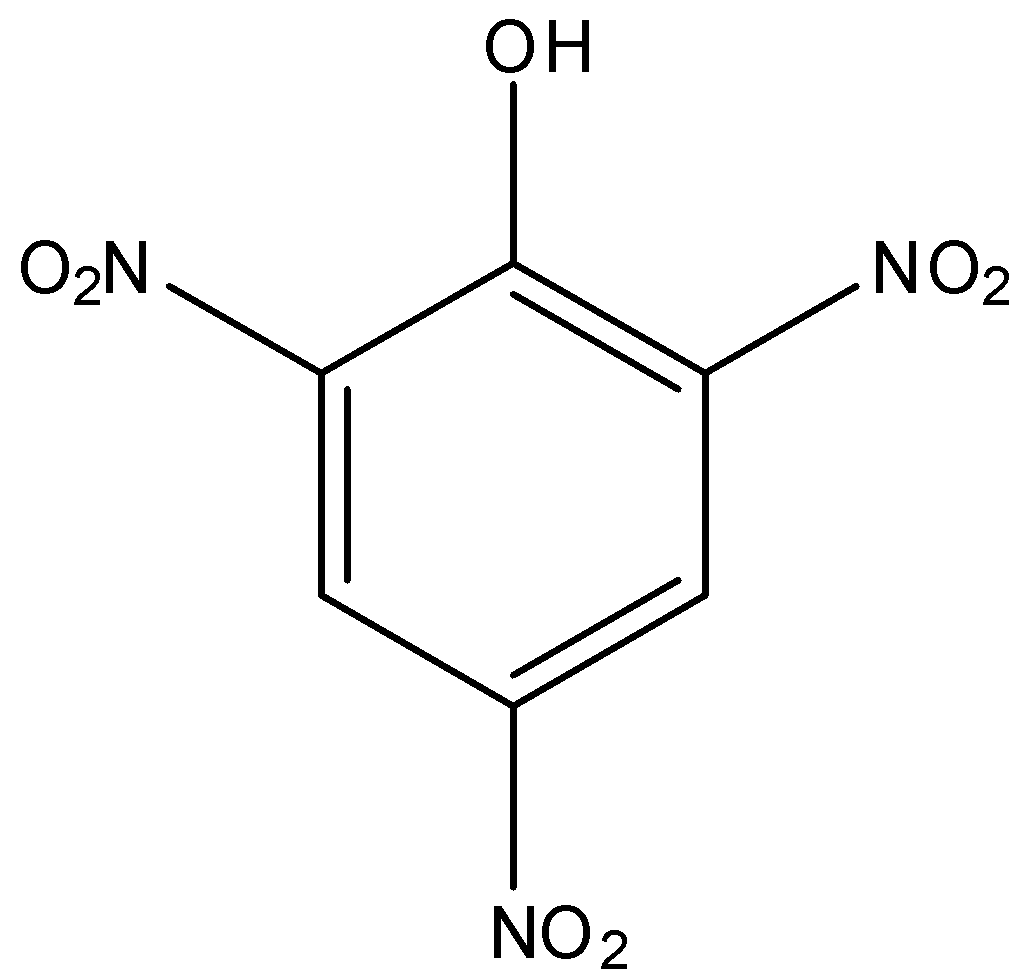
As, we can see there is no carboxyl functional group present in the structure.
Let’s discuss remaining options also. Option B is ethanoic acid. The structure of ethanoic acid is,
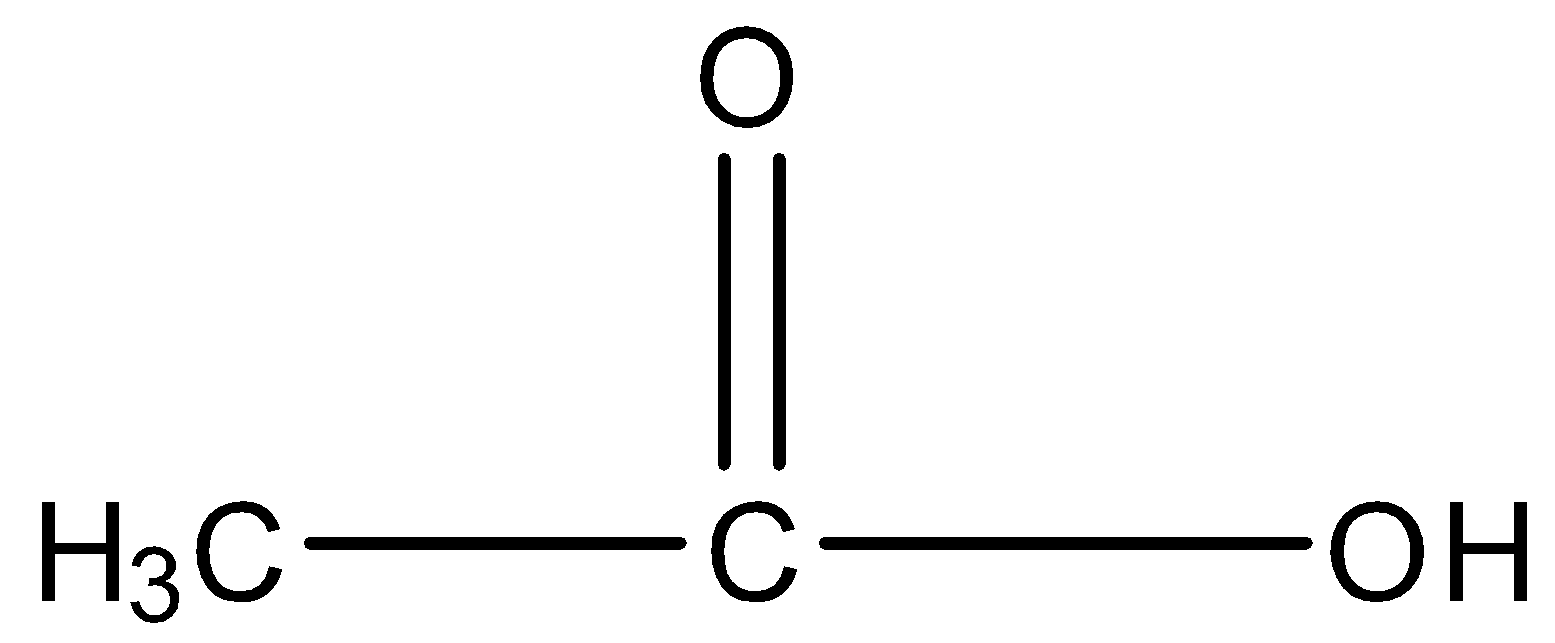
So, ethanoic acid has a carboxyl functional group.
Option C is salicylic acid. The structure of salicylic acid is,
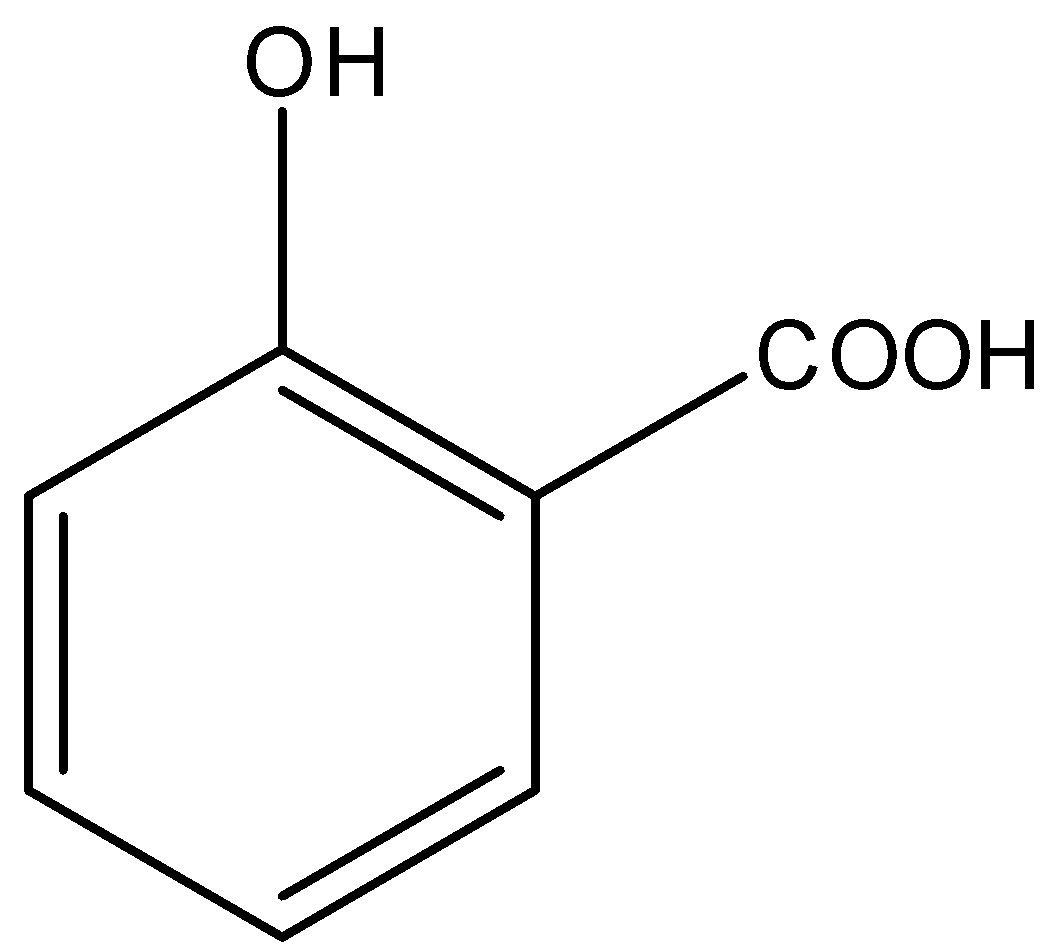
Here also, a carboxyl functional group is present.
Option D is benzoic acid. We know that benzoic acid is the compound formed when a hydrogen atom of benzene is replaced by a carboxyl group. The structure of benzoic acid is,
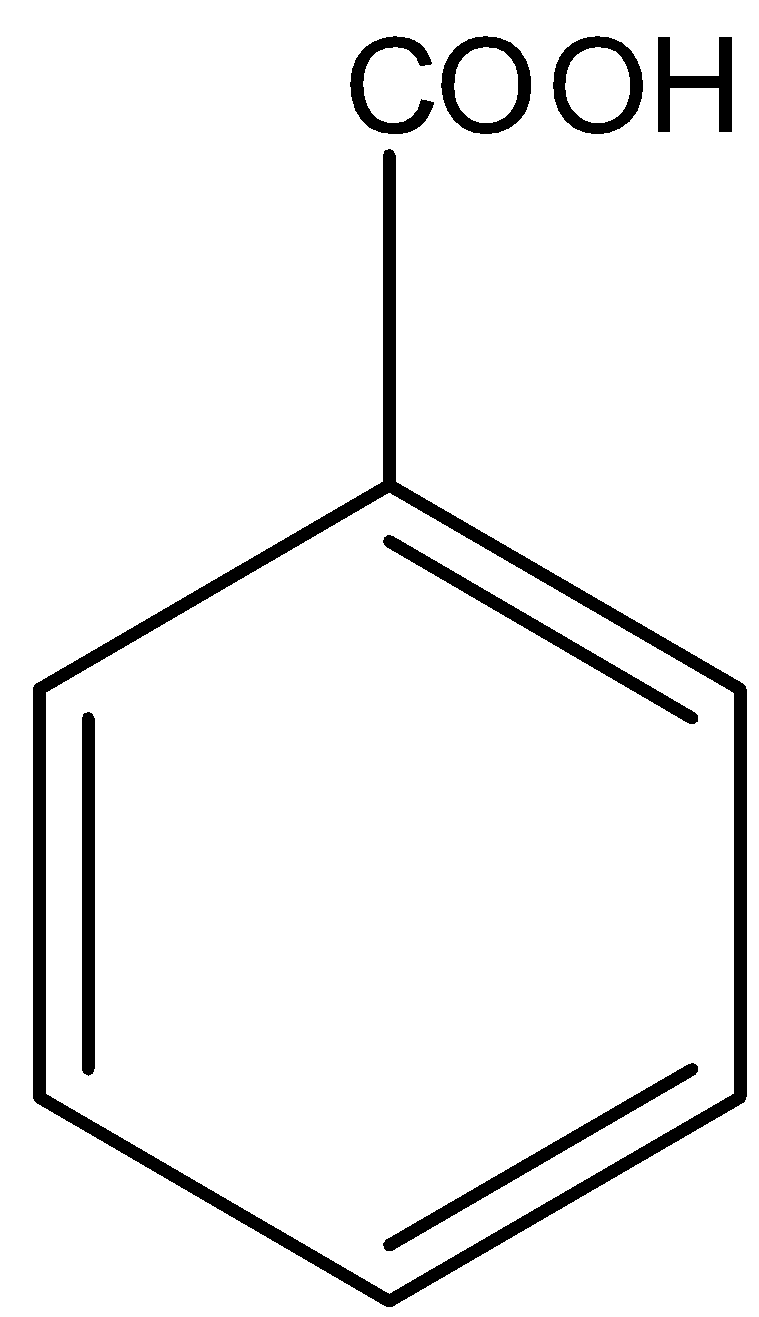
Here also, the carboxyl group is present.
So, among the options only picric acid does not have a carboxyl group.
Hence, the correct choice is option A.
Note: Carboxyl group undergoes ionization by releasing hydrogen atom from the hydroxyl group (OH). Thus, the carboxyl group is called good acids. When the hydrogen atom leaves from the hydroxyl group, the oxygen has a negative charge which it shares with the second oxygen atom in the group. This allows the carboxyl to remain stable on oxidation.
