Question
Question: Which disaccharide is present in milk? A.Maltose B.Galactose C.Sucrose D.Lactose...
Which disaccharide is present in milk?
A.Maltose
B.Galactose
C.Sucrose
D.Lactose
Solution
A disaccharide is a saccharide or carbohydrate which upon hydrolysis gives two molecules of the same monosaccharide or different monosaccharides.
The general formula of disaccharides is C12H22O11 . For example, sucrose, lactose, maltose etc. are disaccharides.
Complete step by step answer:
Milk primarily consists of fat, protein, lactose and water.
Lactose occurs in milk and this is the reason why it is also called milk sugar. Human milk contains 6 to 8 percent lactose and cow’s milk contains 4 to 5 percent lactose.
Lactose is a disaccharide because on hydrolysis with either an acid or an enzyme, it gives two molecules of different monosaccharides glucose and galactose. This is shown below:
LactoseC12H22O11+H2OH + orLactaseGlucoseC6H12O6+GalactoseC6H12O6
The two monosaccharides units are joined together via an ethereal or oxide linkage formed by the loss of a molecule of water. Such a linkage between two monosaccharide units, i.e., glucose and galactose in case of lactose, through oxygen atom is called glycosidic linkage.
In case of lactose, the carbonyl group of the glucose unit is free and so it is a reducing sugar. Thus, it forms an osazone, undergoes mutarotation and also reduces Tollen’s reagent or Fehling’s solution.
According to methylation studies, both glucose and galactose are present in the pyranose form.
It also reveals that glucose is the reducing half while galactose is the non-reducing half. Moreover, C-1 of the galactose unit is attached to C-4 of the glucose unit.
Since the enzyme called emulsion, which specifically hydrolyses β -glycosidic linkages also hydrolyze lactose, therefore, galactose must be present in the β -form.
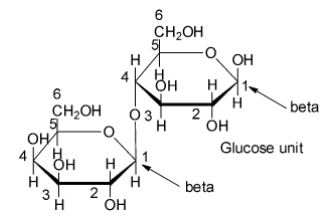
Thus, the disaccharide present in milk is lactose and so option D is correct.
Note:
The proteins are mostly casein proteins. One end of these proteins likes to be surrounded by water and hates to be surrounded by fats while the other end hates to be surrounded by water and likes to be surrounded by fats.
The casein proteins form micelles which are tiny spheres, with the water-hating ends facing inside and the water-loving ends facing outwards into the water.
Each end of the protein which is sticking into water grabs electrons and will attain a negative charge. Thus, all the micelles will be negatively charged and so they will repel one another which prevents them from aggregating or clumping together.
