Question
Question: Which d-orbital has a different shape from the rest of all d- orbitals? A.\({{\text{d}}_{{{\text{...
Which d-orbital has a different shape from the rest of all d- orbitals?
A.dx2 - y2
B.dz2
C.dxz
D.dxy
E.dyz
Solution
The s shell has only 1 orbital. Therefore, it can hold only 2 electrons. It is spherical. P shell has 3 orbitals and holds a maximum of 6 electrons. Each P orbital has a pair of lobes on either side.
The d shell has 5 orbitals in which except 1 orbital, all others have 4 lobes.
Complete step by step answer:
Let us look at all the orbitals of d.
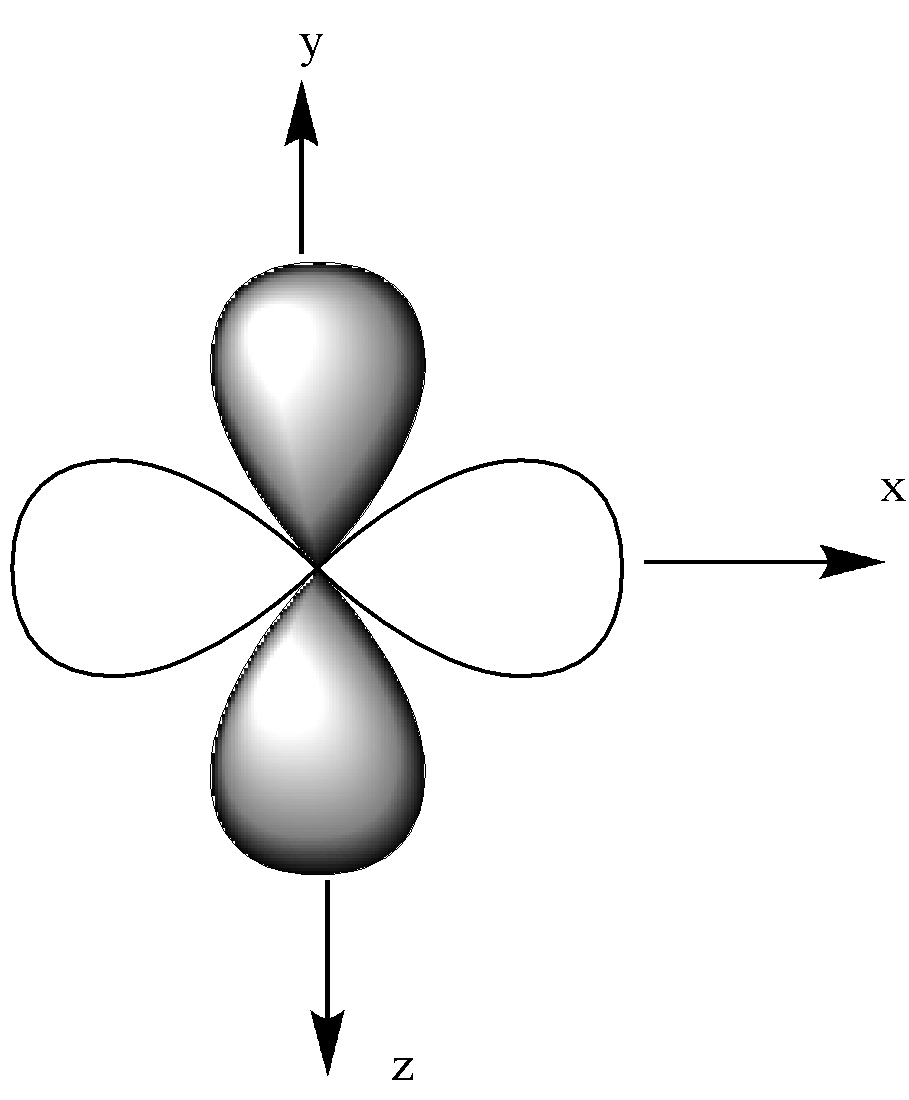
We know that there are 5 lobes in the d orbital. They are dxy, dxz, dyz, dx2 - y2, dz2
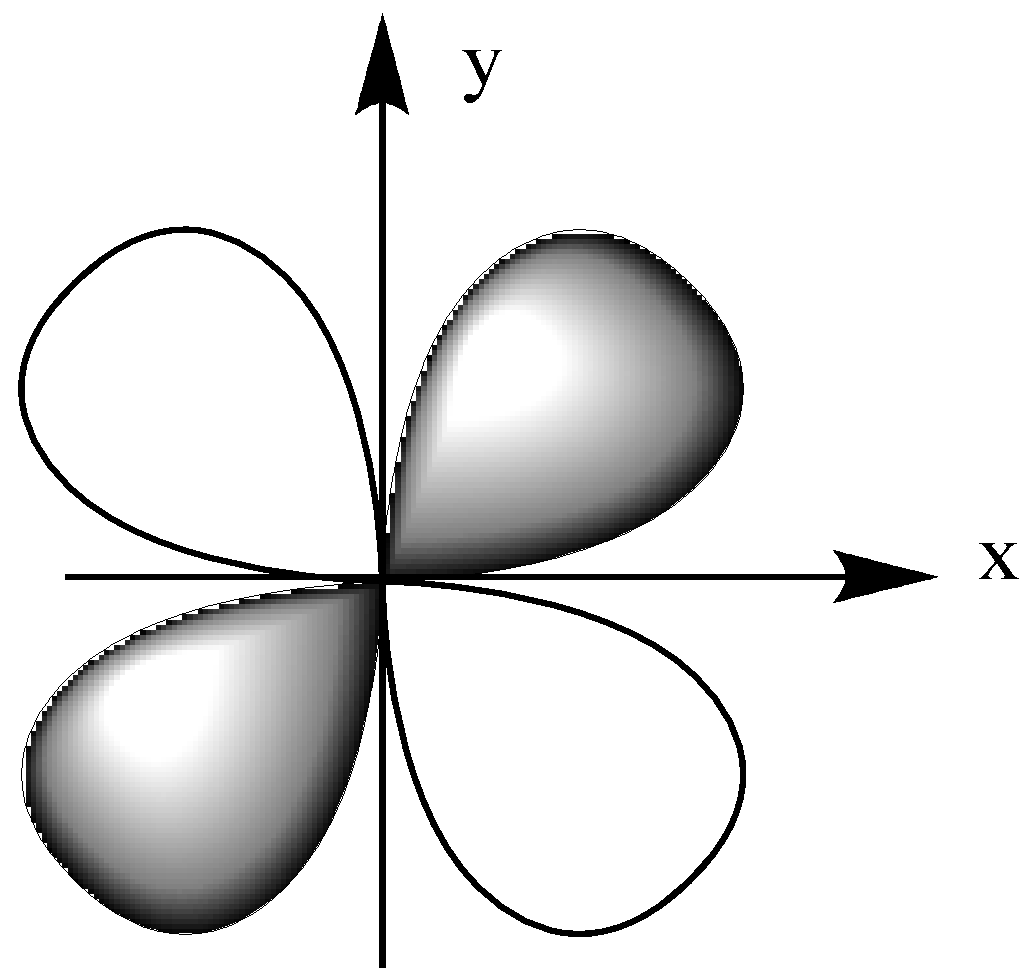
First, let us have a look at the orientation of the dxy orbital. It is in the x-y plane.
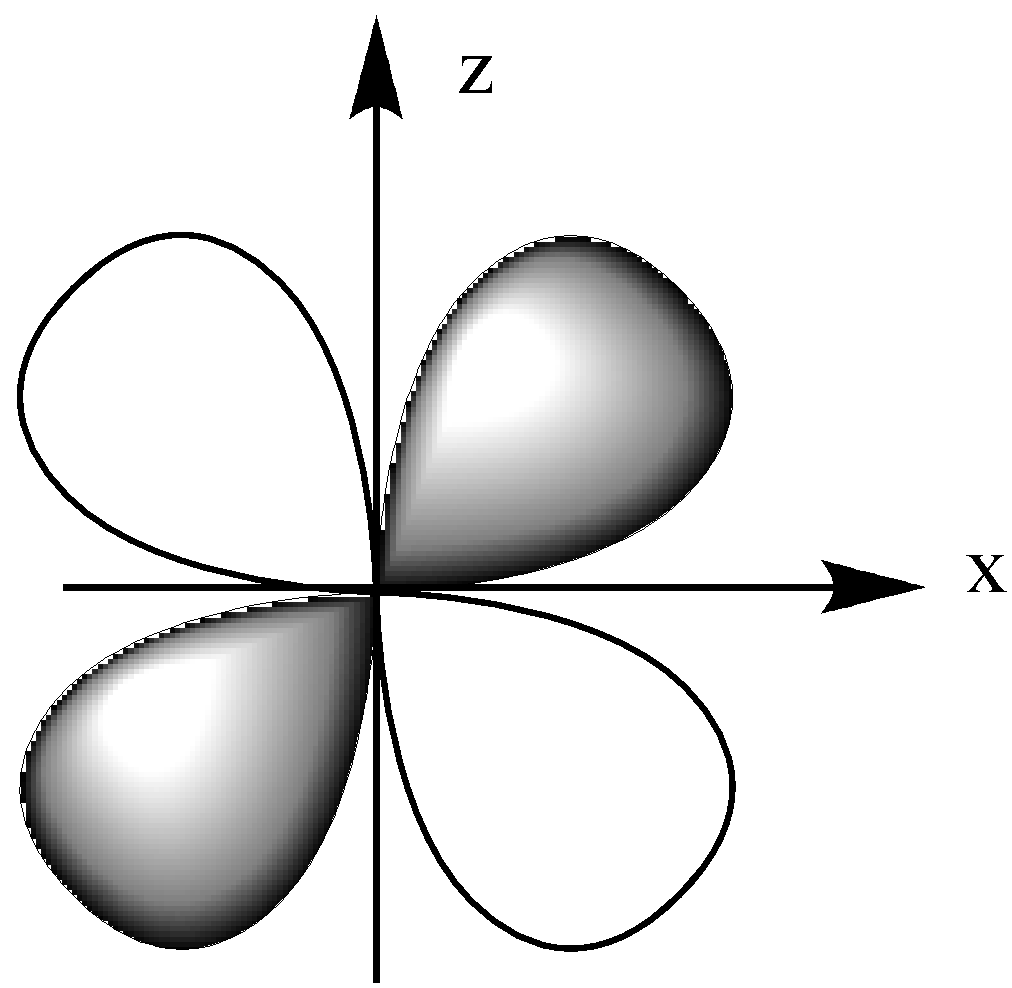
The dxz orbital is in the x-z plane, which means the lobe lies between the x and y-axis.
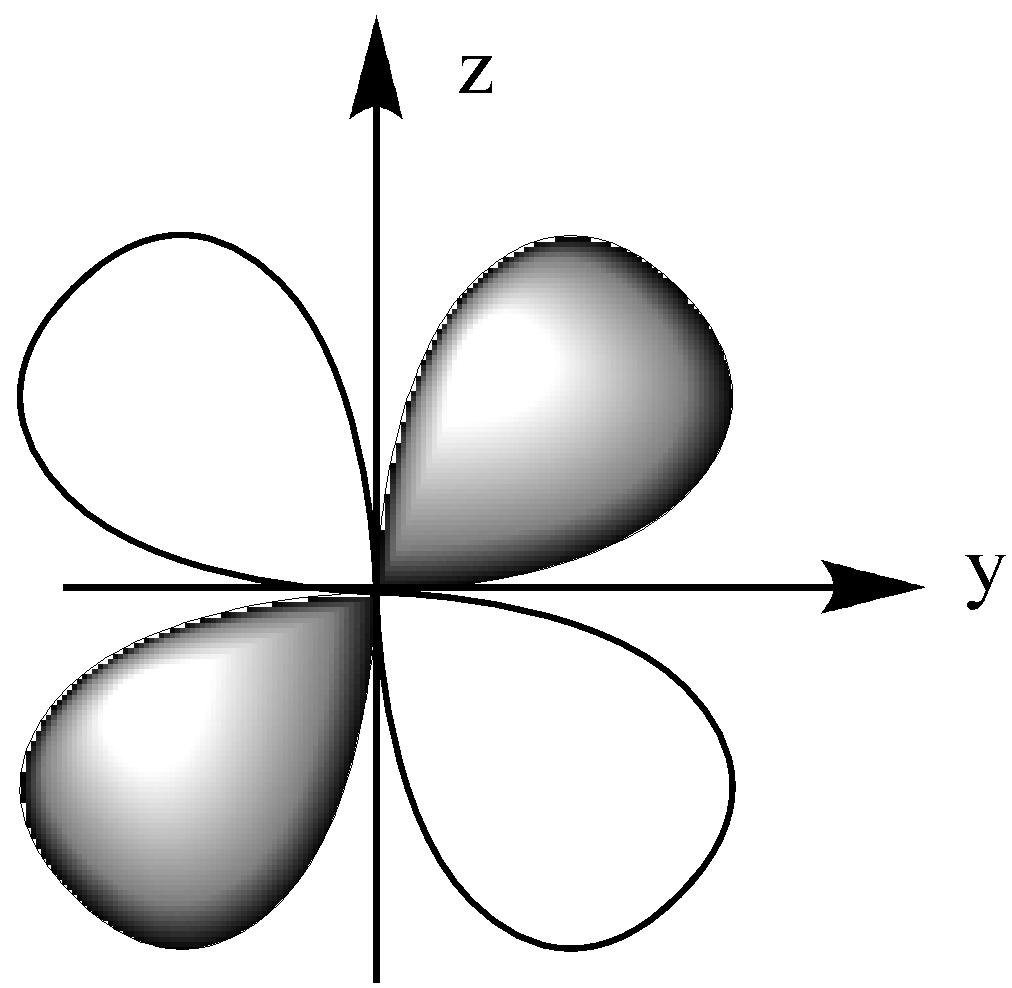
The dyz orbital lies in the y-z plane, which is between the z and y-axis.
The dx2 - y2 orbital lies on the x and y-axis.
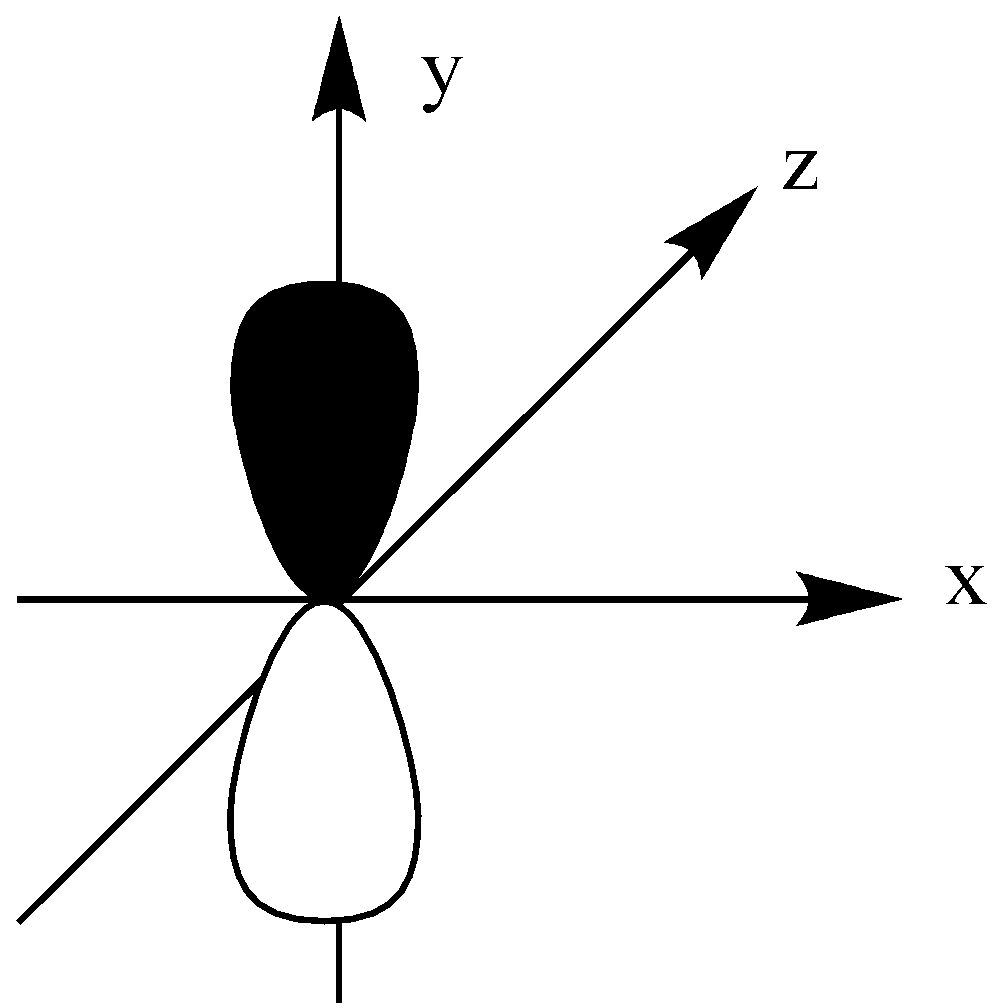
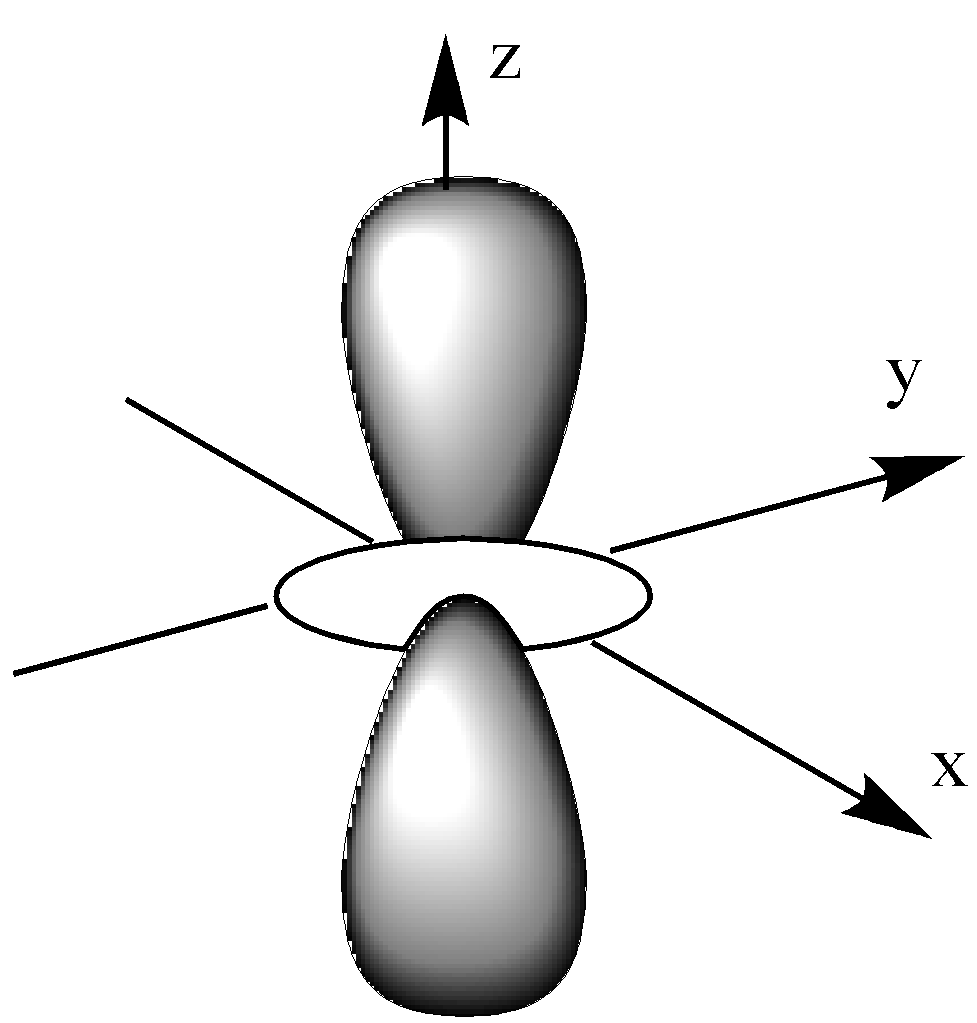
The dz2 orbital is like a single dumbbell along the z-axis and a ring around the nucleus on the XY plane. It has only 2 lobes and 1 ring.
We could see that all other orbitals except the dz2 orbital have a double dumbbell shape, 4 lobes.
dz2 have single dumbbell-shaped and 2 number lobes.
Thus the correct answer is dz2
Therefore, the correct option is (B) .
Note: The shapes of these orbitals help us to find the d orbital splitting on the approach of octahedral as well as tetrahedral ligands. The Crystal Field theory can be understood well with the knowledge of the structure of the orbitals and their orientation. Transition metals show special properties because of the d orbitals.
