Question
Question: Which compound of xenon is not possible? A. \(Xe{F_2}\) B. \(Xe{F_4}\) C. \(Xe{F_5}\) D. ...
Which compound of xenon is not possible?
A. XeF2
B. XeF4
C. XeF5
D. XeF6
Solution
Xenon is a chemical element with atomic number 54 .It can only combine with an even number of F atoms to form xenon fluorides and not with odd numbers of F atoms.
Complete step by step answer:
Xenon is an inert gas. Its electronic configuration is [Kr]4d105s25p6 . All orbitals that are filled have paired electrons.
Xenon can combine with even number of F atoms to form XeF2 XeF4 and XeF6 .This is because the promotion of 1, 2, or 3 electrons from the 5p filled orbitals to 5d vacant orbitals will give rise to 2,4,6 half-filled orbitals. The structures are as shown:
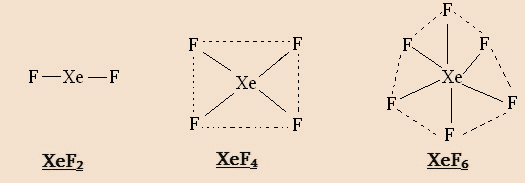
It cannot combine with an odd number of F –atoms.
Thus, the formation of XeF3 and XeF5 is not possible.
Hence, option C is correct.
Note:
Xenon is obtained commercially as a by-product of the separation of air into oxygen and nitrogen. It is 4.5 times heavier than Earth’s atmosphere (which consists of a mixture of a number of gaseous elements and compounds). Its mass comes from its nucleus, which contains 54 protons and a varying (but similar) number of neutrons.
