Question
Question: Which compound is used for bleaching clothes in the laundry? (A) Bleaching powder (B) Washing ...
Which compound is used for bleaching clothes in the laundry?
(A) Bleaching powder
(B) Washing powder
(C) Baking powder
(D) Plaster of Paris
Solution
bleaching is a process used for the removal of colouring matter by converting into the colourless matter. The substrate is oxidized by the bleaching agent. The bleaching agent is capable of supplying the chlorine. The chlorine on the action of acid generates the nascent oxygen which oxides the clothes in the laundry.
Complete step by step answer:
Bleaching is a chemical treatment used for the removal of colouring matters from the substrate. In the laundry, the bleaching agent is a chemical treatment to whiten the clothes or decolorized clothes.
A bleaching agent is a chemical that whitens or decolorizes the fabric. This destroys the chromophore (Colour bearing groups) through the oxidation or the reduction of these groups.
One of the common bleaching agents is calcium hypochlorite CaO(Cl)2 . It is an inorganic substance and it’s a main component of the commercially used bleaching powder, chlorine powder, or chlorinated lime for the water treatment and as a bleaching agent. The CaO(Cl)2 is a stable compound and it supplies the oxygen for the bleaching action.
The CaO(Cl)2 is an oxidizing agent that produces oxygen for bleaching.
The bleaching powder when treated with the dilute acid produces the nascent oxygen [O] . The nascent oxygen oxidizes the colouring matter and whitens the substrate.
The structure of the bleaching powder is as shown below:
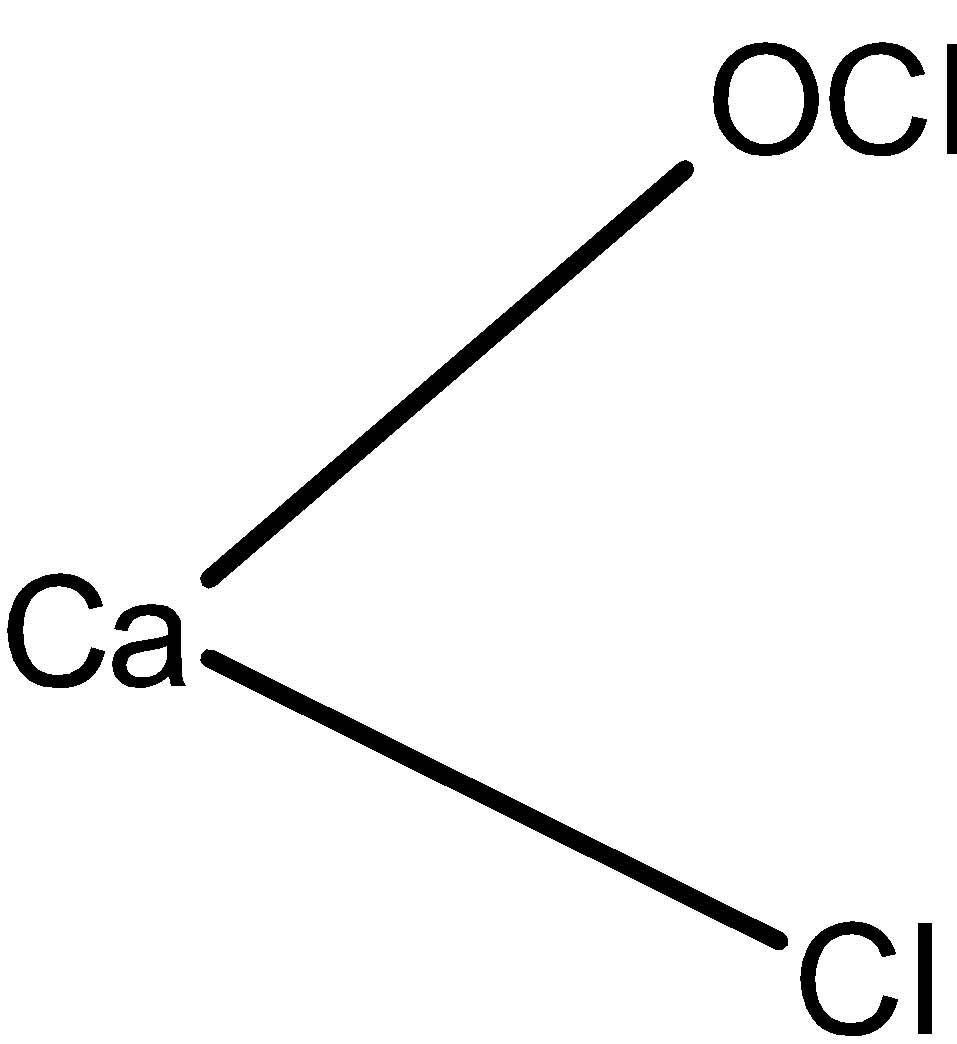
The CaO(Cl)2 calcium hypochlorite is also known as the bleaching powder.
Hence, (A) is the correct option.
Additional information:
The bleaching action of the calcium hypochlorite is as shown below:
The bleaching power CaO(Cl)2 is a mixed salt of hydrochloric acid and hypochlorous acid. When bleaching powder is treated with the dilute aid, it gives the nascent oxygen [O] .the reaction is as shown below,
