Question
Question: Which compound is formed when a mixture of calcium acetate and calcium formate is heated? A.
B.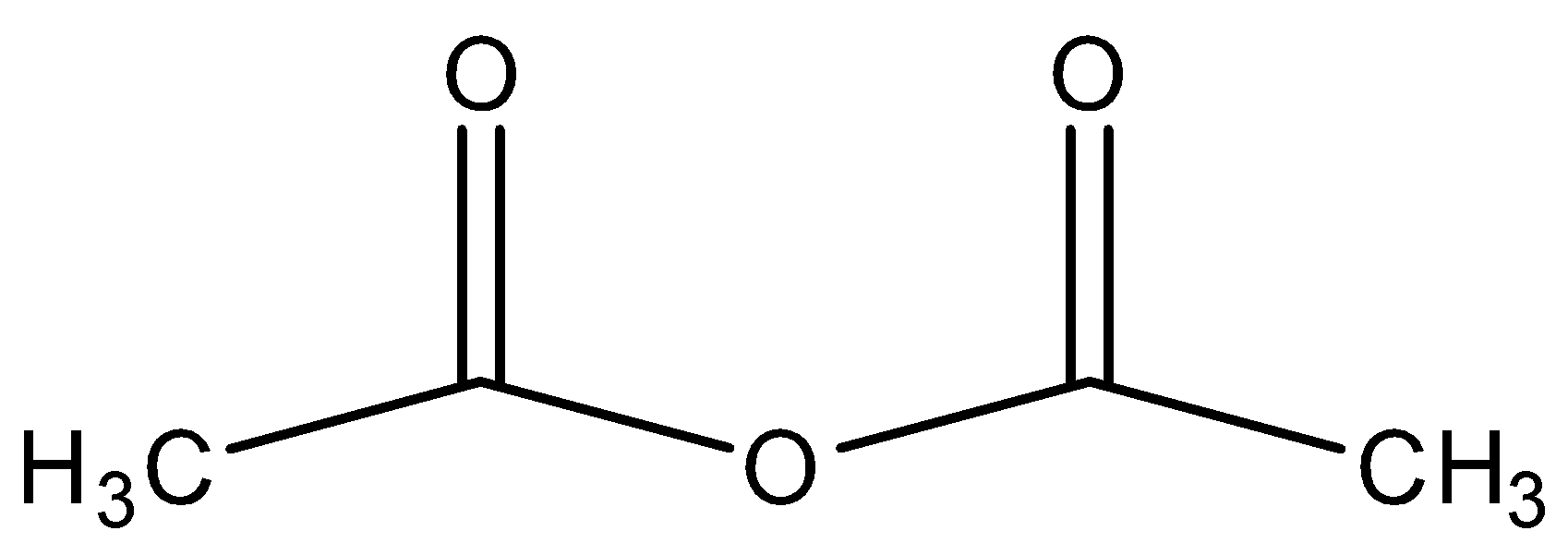
C.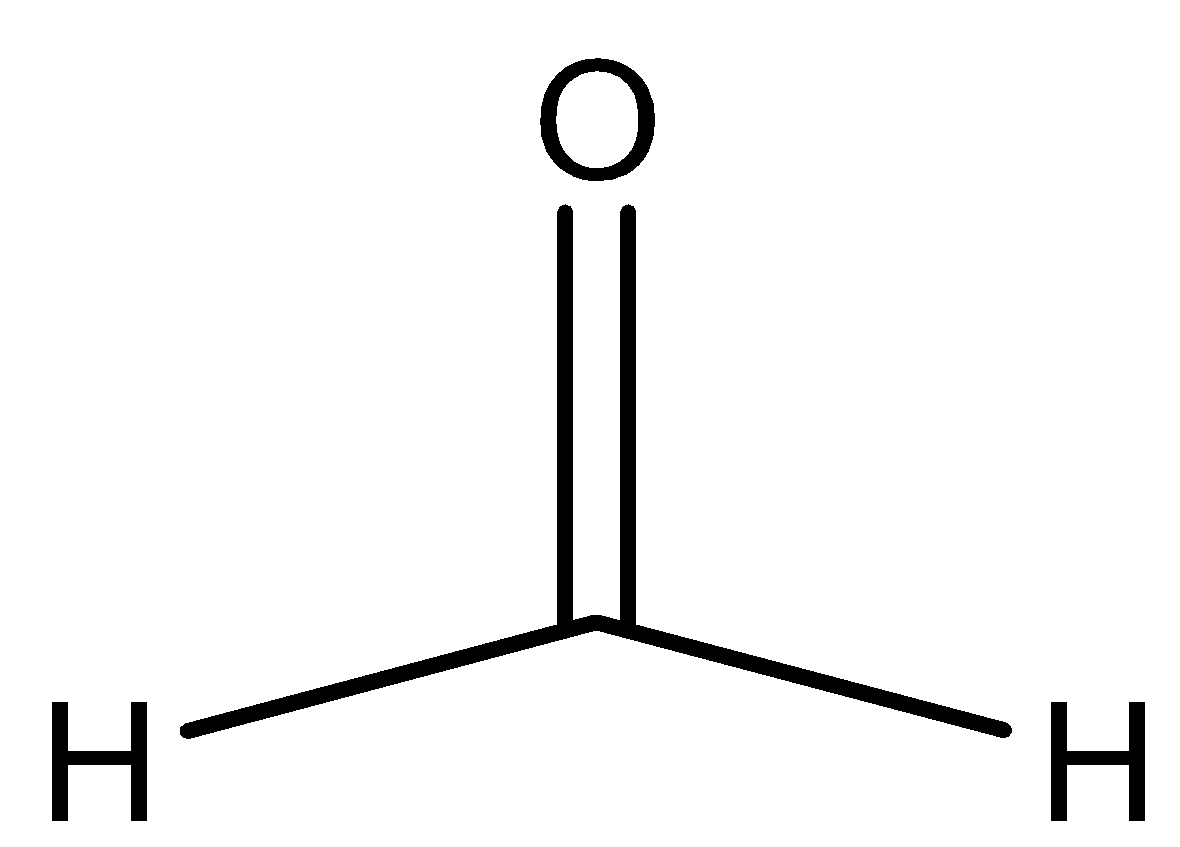
D.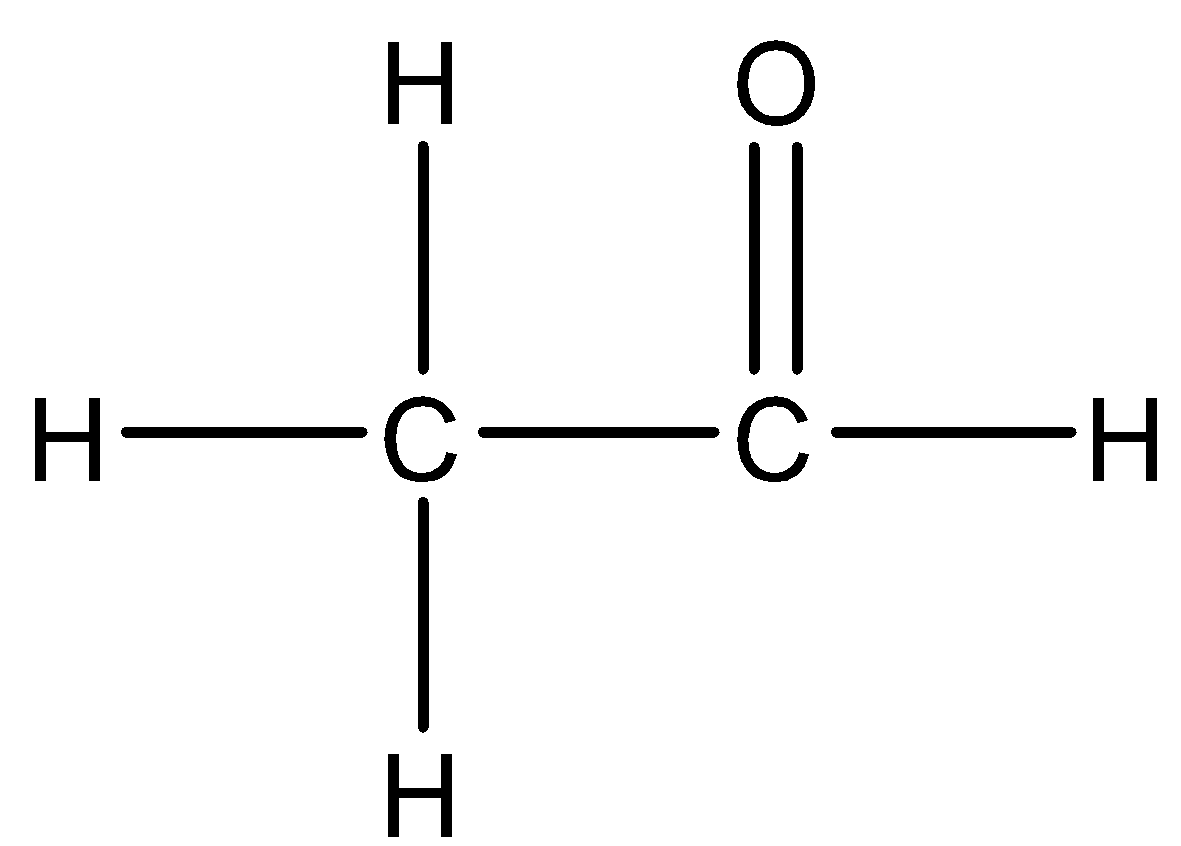
Solution
Calcium acetate is a chemical compound which is a calcium salt of acetic acid. Its formula is Ca(C2H3O2), whereas calcium formate is the calcium salt of formic acid. Its formula is Ca(HCOO)2.
Complete answer:
When a mixture of calcium acetate and calcium formate is heated, acetaldehyde is formed.
The equation is as shown:
Ca(−OCOCH3)2+Ca(−OCOH)2Δ2H3C−CHO+2CaCO3
Acetaldehyde is one of the most important aldehydes, occurring widely in nature and being produced on a large scale in industry.
Its boiling point is 20.2∘C and melting point is −123.5∘C.
Hence, option D is correct.
Additional information:
Acetaldehyde is widely used in the manufacturing of perfumes, drugs, acetic acid, flavoring agents, dyes etc. It is toxic when applied externally for prolonged periods. It was first observed in the year 1774 by the Swedish pharmacist/chemist Carl Wilhelm Scheele. In the whole world, China is the largest consumer of acetaldehyde.
Note:
All ethyl alcohols which are broken down in the human body are first converted to acetaldehyde, and then this acetaldehyde is converted into acetic acid radicals, also known as acetyl radicals. Acetaldehyde is a poison which is a close relative of formaldehyde.
