Question
Question: Which compound is a secondary alcohol? A.Butan-1-ol B.Butan-2-ol C.Isobutyl alcohol D.2-meth...
Which compound is a secondary alcohol?
A.Butan-1-ol
B.Butan-2-ol
C.Isobutyl alcohol
D.2-methylpropan-2-ol
Solution
Before solving this question, we should know what are secondary alcohols and the basic difference between the primary alcohol, secondary alcohol and the tertiary alcohol.
Complete answer:
A secondary alcohol is where the alcohol is placed on the carbon which is bonded to two other carbon atoms. The alcohol groups are those that in a compound there is at least one hydroxyl group attached. The hydroxyl group is the basic functional group of the compound.
The alcohols groups are divided into three types on the basis of the total number of alkyl groups which are connected to the carbon connected to the hydroxyl group.
The simplest primary alcohol is CH3OH i.e. methanol, the simplest secondary alcohol is 2-propanol and the simplest tertiary alcohol is 2-methylpropan-2-ol.
Among the following options only butan-2-ol is a secondary alcohol, because in the structure of butan-2-ol the alcohol is placed on the carbon which is bonded to two other carbon atoms. The structure of butan-2-ol is mentioned below:
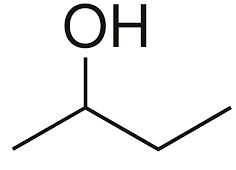
Hence, the correct answer is option (B) i.e. butan-2-ol is the secondary alcohol.
Note:
Many chemical tests are also used to distinguish between the primary alcohol, secondary alcohol and tertiary alcohol. A chemical test called Lucas test is also used for the same purpose. The primary alcohol does not respond to the test, the secondary alcohol takes a bit of time and after that gives the result while the tertiary alcohol reacts immediately as come in contact with the Lucas reagent.
