Question
Question: Which compound in the above pair undergoes faster \({{\text{S}}_{\text{N}}}{\text{1}}\) reaction? ...
Which compound in the above pair undergoes faster SN1 reaction?
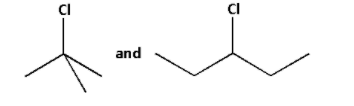
Solution
A unimolecular nucleophilic substitution reaction in which the rate of reaction depends on only one reactant is known as SN1 reaction. Identify if the given halides are primary, secondary or tertiary and then decide which of them reacts faster.
Complete step by step answer:
The given halides are as follows:
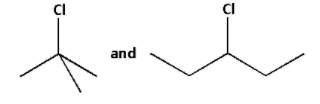
The simplified forms of the given halides are as follows:
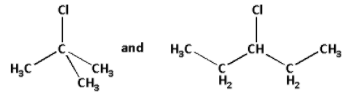
In the first halide, the carbon atom to which the chlorine atom is attached to three more carbons. Thus, the first halide is a tertiary halide.
In the second halide, the carbon atom to which the chlorine atom is attached to two more carbons. Thus, the second halide is a secondary halide.
When the chloride group leaves the structure, a carbocation will be formed. The tertiary halide will form tertiary carbocation and the secondary halide will form secondary carbocation.
The carbocation having more number of adjacent carbon atoms is the most stable. Thus, the tertiary carbocation is more stable.
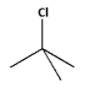
The tertiary alkyl halide leads to the formation of a more stable tertiary carbocation. Thus, the tertiary alkyl halide undergoes a faster SN1 reaction.
Thus, the compound that undergoes faster SN1 reaction is,
Note: The order of stability of alkyl halides is as follows:
Tertiary halide > secondary halide > primary halide
The order of stability of carbocations is as follows:
Tertiary carbocation > secondary carbocation > primary carbocation
Thus, a tertiary halide prefers a SN1 reaction. And a primary halide prefers a SN2 reaction. A bimolecular nucleophilic substitution reaction in which the rate of reaction depends on two reactants is known as SN2 reaction.
