Question
Question: Which compound has planar structure? (A) \(Xe{F_4}\) (B) \(XeO{F_2}\) (C) \(Xe{O_2}{F_2}\) (...
Which compound has planar structure?
(A) XeF4
(B) XeOF2
(C) XeO2F2
(D) XeO4
Solution
Any compound is said to be planar only when all atoms of a molecule lie in the same plane. Here, we first calculate the hybridization of the given compound and then we will predict the shape based on the hybridization obtained.
Complete step by step answer:
XeF4 is known to have square planar structure.
At first we will calculate the hybridisation of XeF4
The central atom is Xe. In the valence shell of Xe, there are six electrons in 5p orbital and 2 electrons in the 5s orbital. There are 5d and 5f orbitals left that are empty.
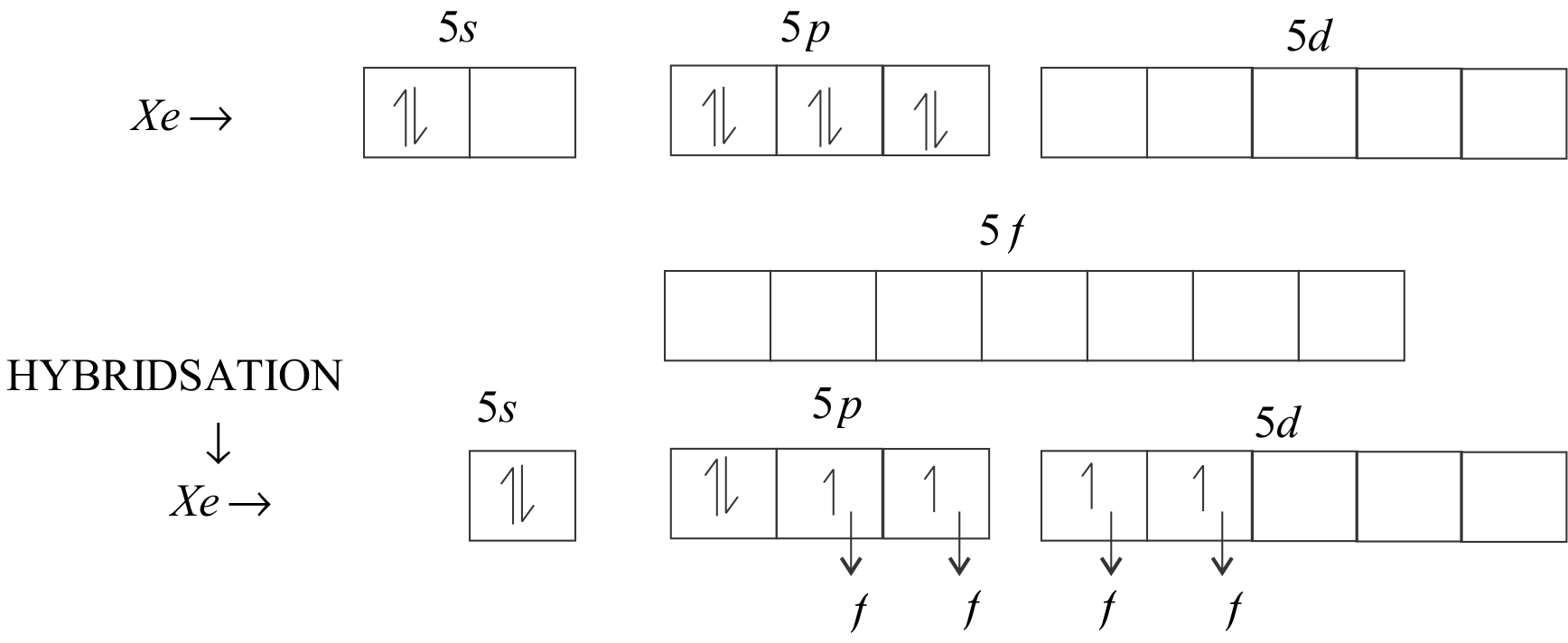
sp3d2 will be the hybridization of XeF4.
So, the hybridisation is sp3d2 which contains two lone pairs and four bond pairs orbitals.
So, the geometry of XeF4
So, the molecule is square planar.
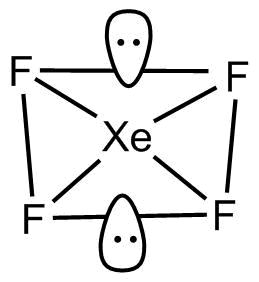
The two lone pairs are present upside down and the other four are bonded with fluorine atoms.
Additional information:
Xenon tetrafluoride is produced as a result for the reaction of xenon with fluorine. The reaction equation can be written as:
Xe + 2F2→XeF4
Xenon tetrafluoride is a colorless crystalline substance. Looking at the structure of xenon tetrafluoride, it has two lone pairs of electrons in addition with the four fluorine ligands in accordance with the VSEPR theory. The lone pairs are actually mutually trans.
Xenon tetrafluoride found to have certain few applications. It is used in reaction with silicon to form simple gaseous products that leaves behind a residue of metal impurities. It has also been used to degrade silicone rubber to analyze the impurities in the rubber.
Note:
It should be noted that the central atom that is xenon has six electron groups out of which the two of them are lone pairs. the hybridization of any atom must be predicted carefully keeping in mind the no. of lone pairs present.
