Question
Question: Which compound gives positive iodoform test? A.2-pentanone B.Pivaldehyde C.Benzaldehyde D.Is...
Which compound gives positive iodoform test?
A.2-pentanone
B.Pivaldehyde
C.Benzaldehyde
D.Isobutyl alcohol
Solution
Hint: Draw the molecular structure of each of the given options and remember positive iodoform test is given by compounds containing methyl ketones and 2-alkanols.

Complete step by step answer:
Iodoform test is used for the identification of aldehyde and ketone.
A positive iodoform test is given by the compounds having CH3CO group in their structure.
When Iodine and sodium hydroxide are added to a compound that contains either a methyl ketone or a secondary alcohol with a methyl group in the alpha position, a pale yellow precipitate of iodoform is formed.
Now we will look at the structure of each of the options provided.
Option (a) 2-pentanone contains a methyl ketone group. Hence, it will give a positive iodoform test.
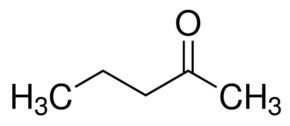
Option (b) Pivaldehyde does not contain methyl ketones and 2-alkanols. That's why it will give a negative test.
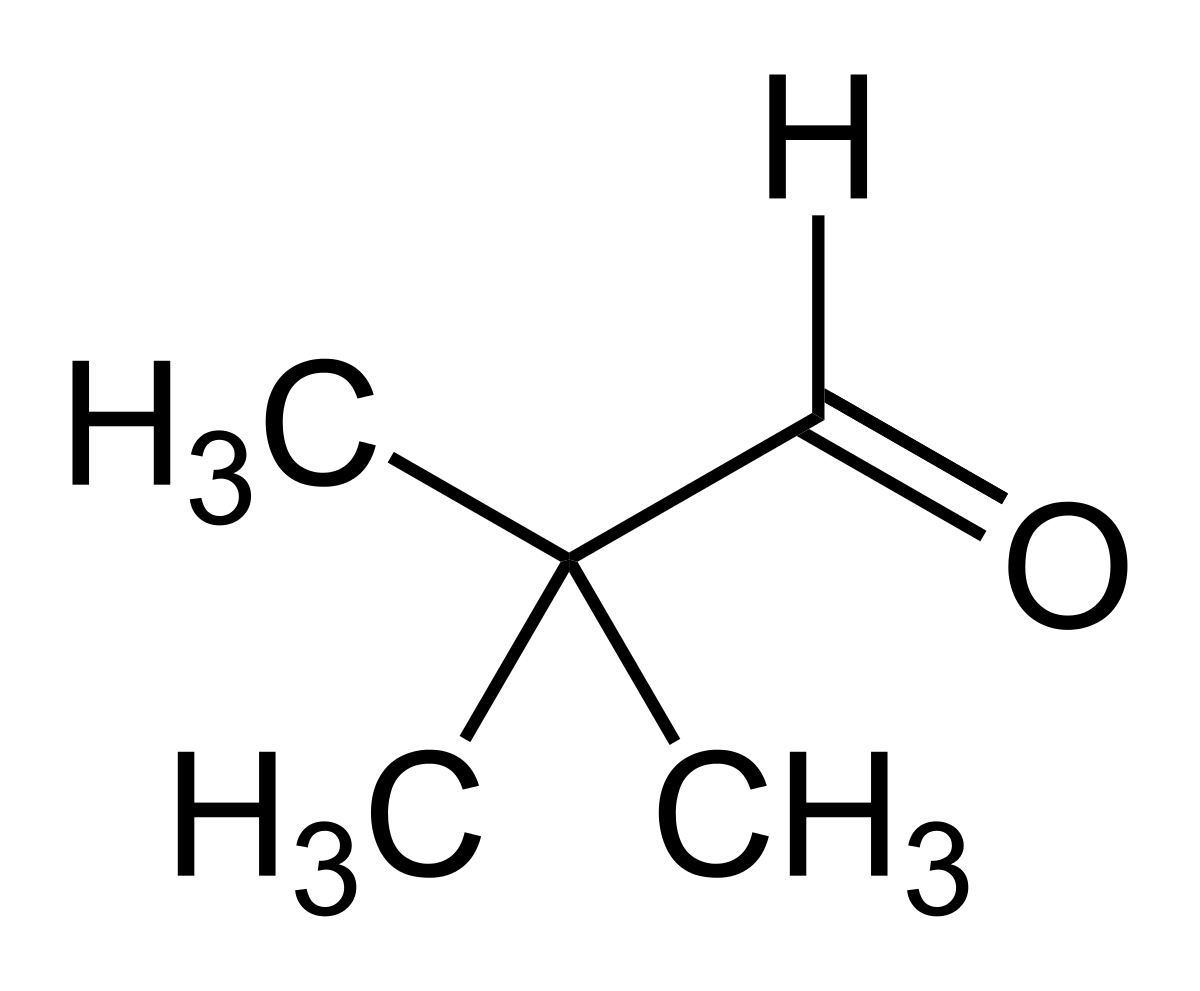
Option (c) Benzaldehyde does not contain methyl ketones and 2-alkanols. That's why it will give a negative test.
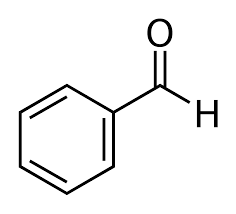
Option (d) Isobutyl alcohol does not contain methyl ketones and 2-alkanols. That's why it will give a negative test.
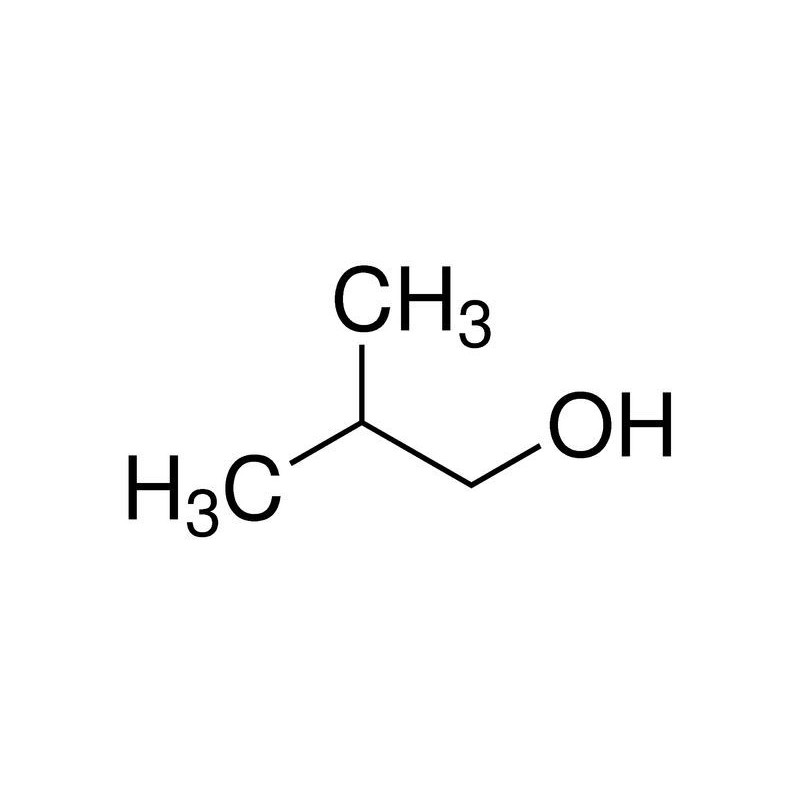
So, correct option is A
Additional information: The pale yellow precipitate of iodoform (CHI3) is formed, which can be identified by its characteristic “antiseptic” smell.
Note: If an aldehyde gives a positive iodoform test, then it must be acetaldehyde since it is the only aldehyde with a CH3CO group.
