Question
Question: Which compound exhibits maximum dipole moment among the following? (A)  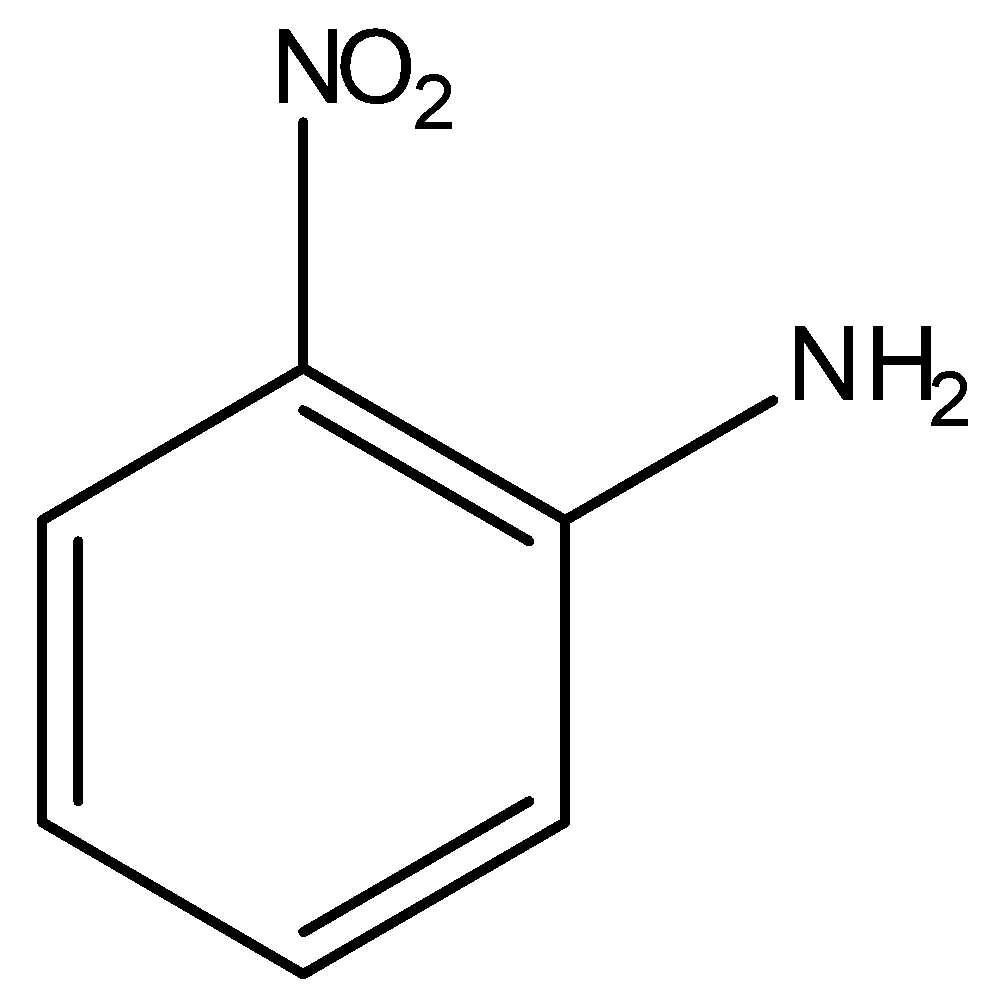
(B) 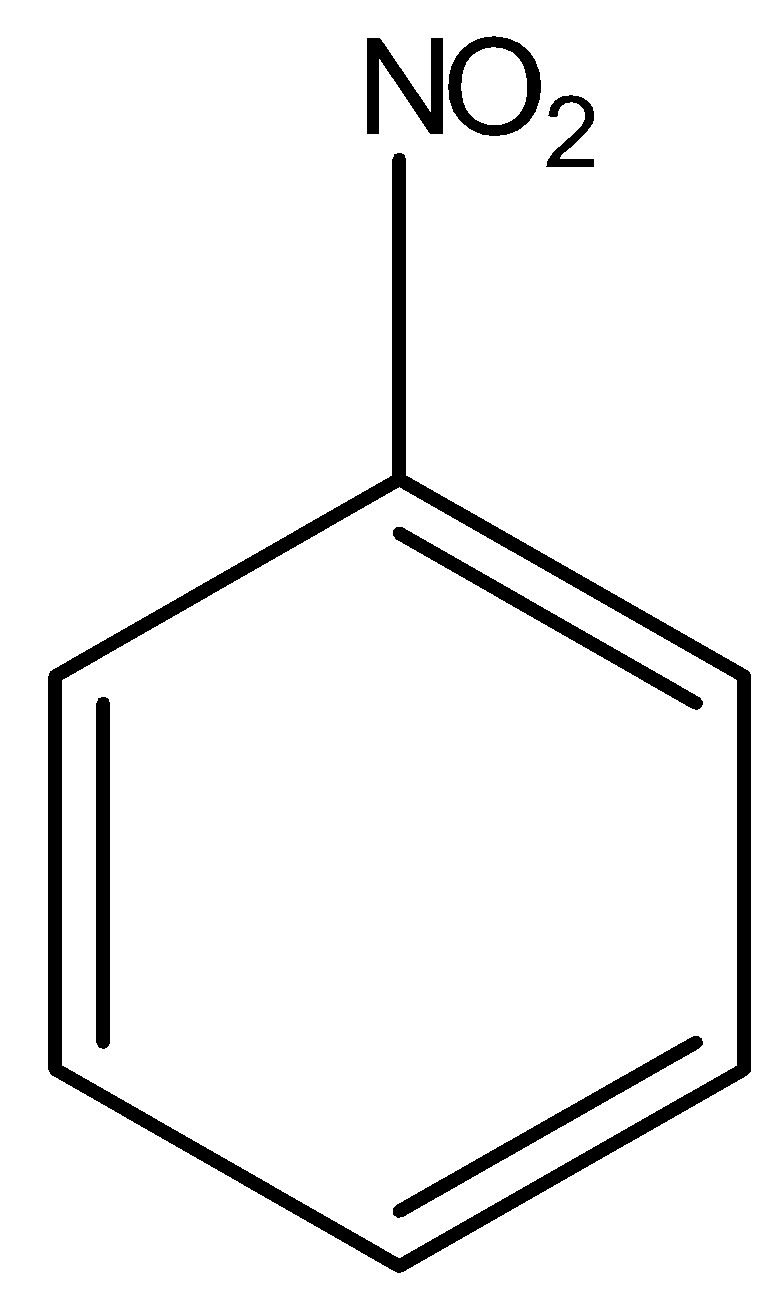
(C) 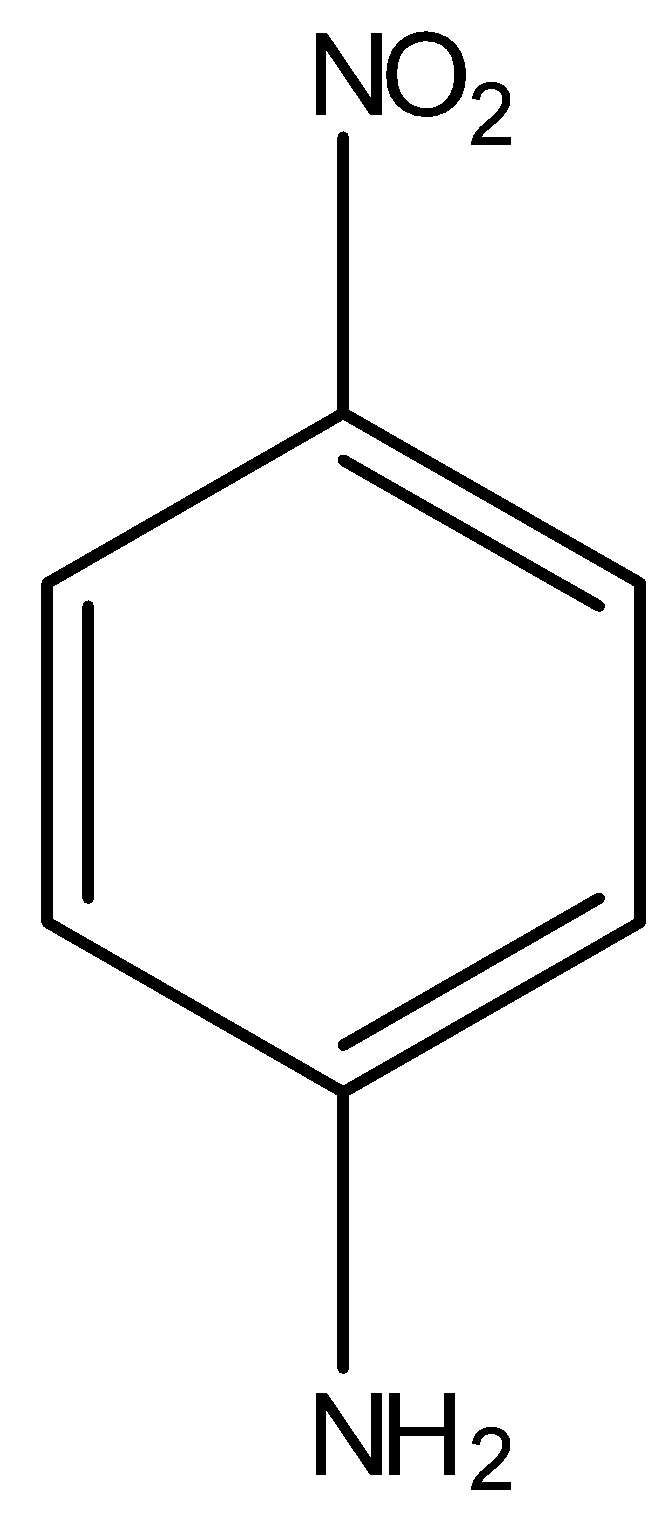
(D) 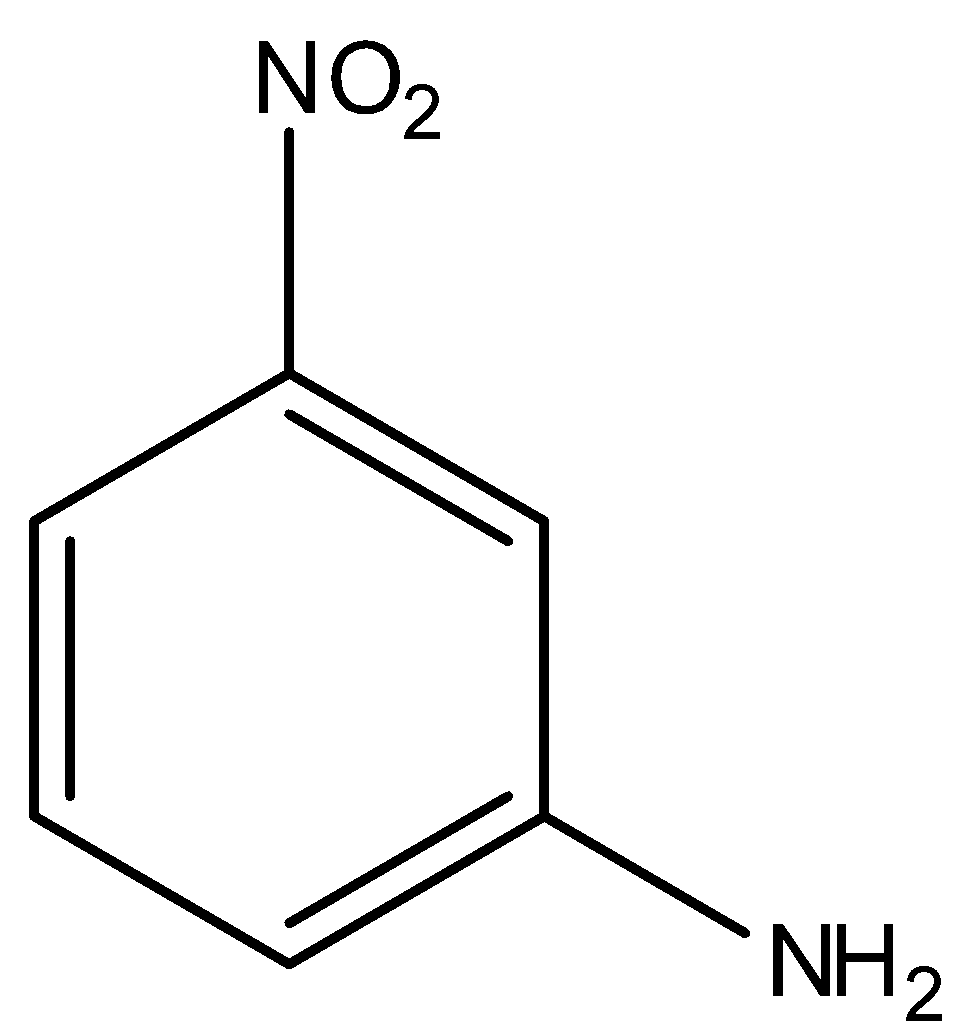
Solution
You must know that NH2 group is an electron donating group while NO2 group is an electron withdrawing group. So, if an NH2 group is present on a ring, then electron density will be towards the ring and when an NO2 group is present on a ring, then electron density will be towards the NO2 group. Dipole moment is the measure of the system's overall polarity.
Complete step by step solution:
Dipole moment, denoted by the symbol μ, arises in any system when there is a separation of negative and positive charges. Separation of charges occurs because of the electronegativity difference between two chemically bonded atoms. Thus, dipole moment is a measure of the polarity of a system. Dipole moment is represented by an arrow with the arrowhead on the negative centre and cross on the positive centre. Arrow symbolises the shift of electron density in the molecule. Now, in the question, we are given nitrobenzene and aniline derivatives basically. Dipole moment in some of the following compounds:
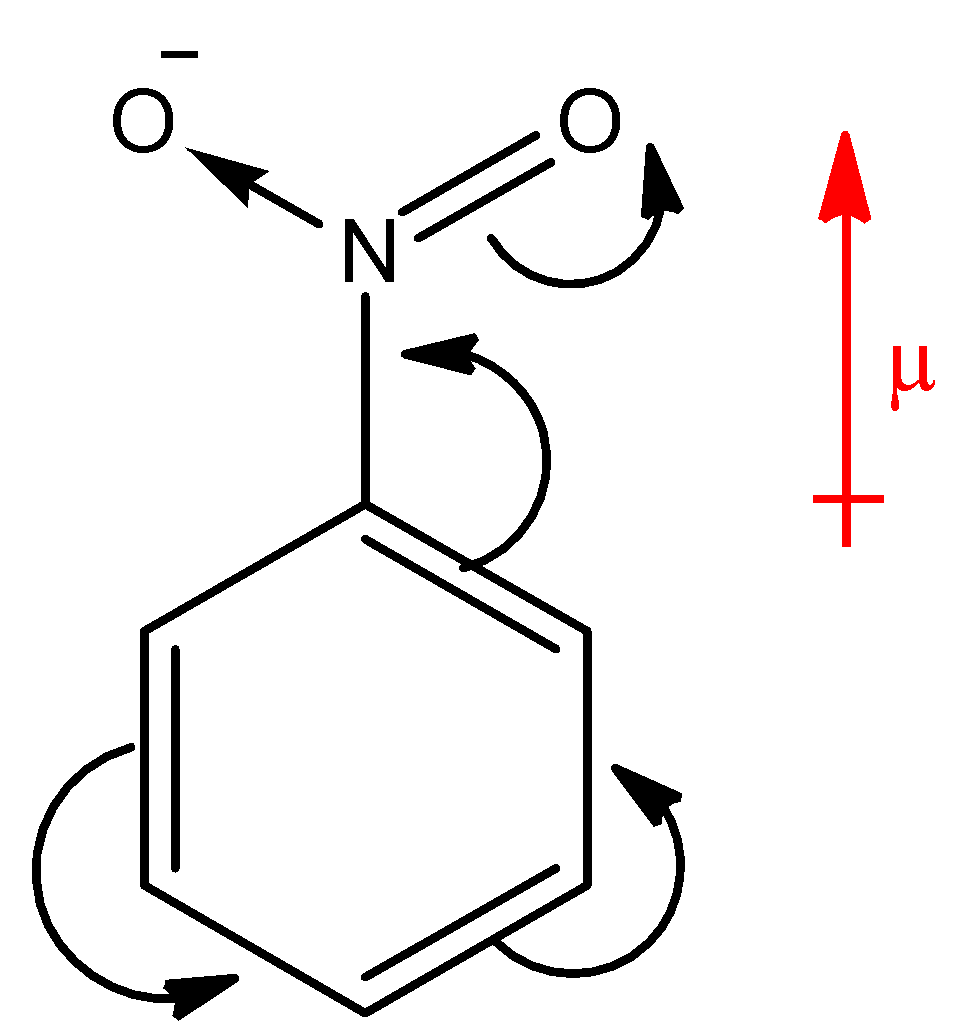
Above compound is nitro benzene. Here, NO2 group is present on a ring which is an electron withdrawing group, hence the electron density will move towards the NO2 group. There will be separation of charges in the system; the ring will acquire partial positive charge and the nitro group will acquire partial negative charge. Hence, the overall dipole moment (μ) of the compound will be in upward direction as shown in the diagram with red arrow. Whereas, the reverse of nitro benzene happens in aniline as shown in the below diagram.
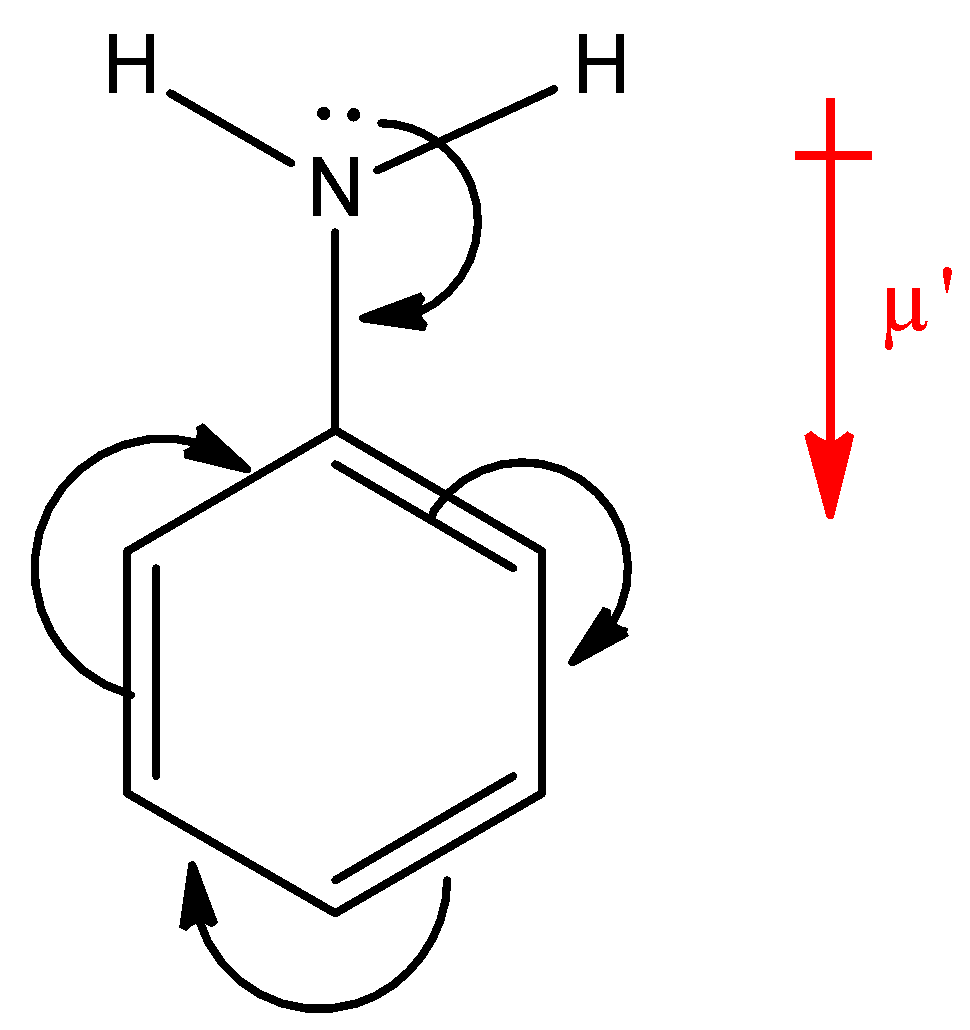
Here, NH2 group is present on a ring, which is an electron donating group, thus the electron density will be towards the ring in aniline. Hence, the dipole moment (μ′) will be downwards. Now, in p-nitroaniline, where both NH2 and NO2 group are present:
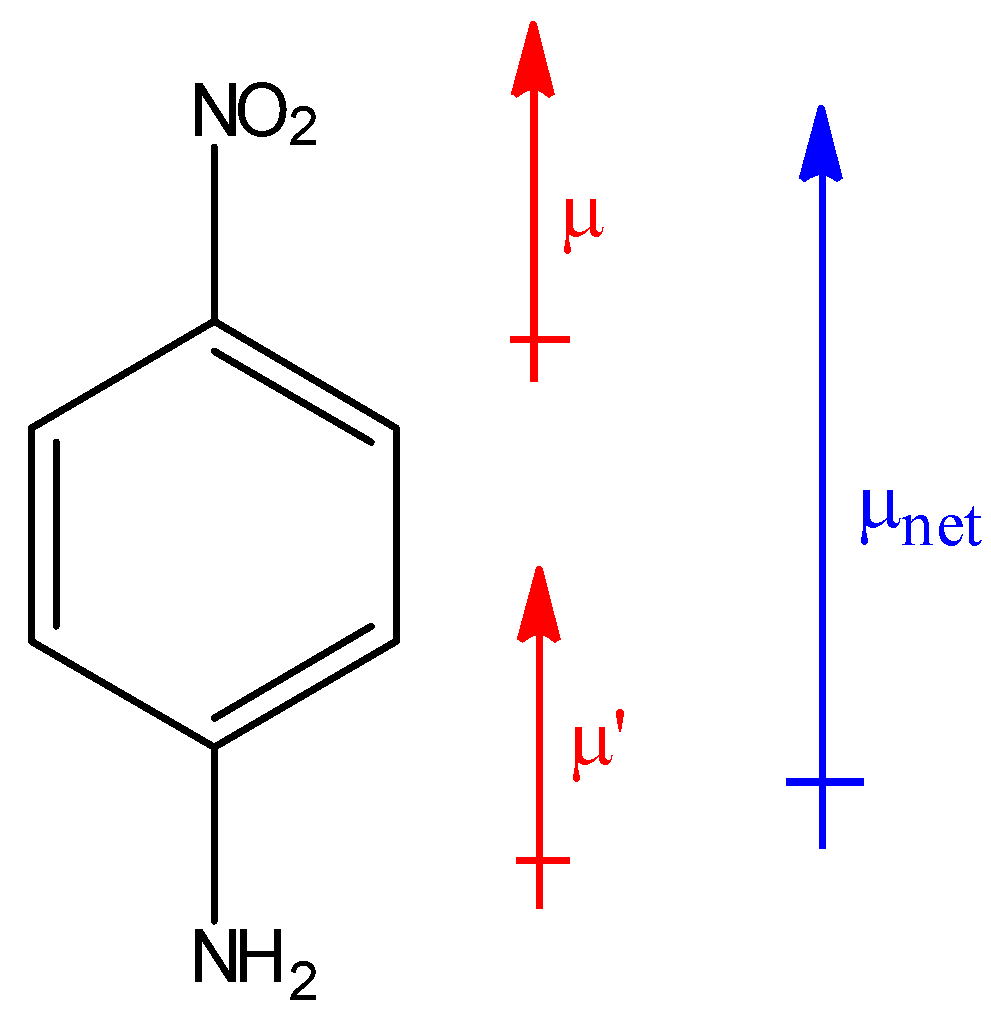
In the above compound, the net resultant dipole moment will be upwards because both individual dipole moments μ and μ′ are in upward direction. Hence, this compound will have the highest dipole moment among all the given benzene derivatives.
Thus, option C is correct.
Note: Dipole moment is a vector quantity since it has both magnitude and direction. The net resultant dipole moment is the vector sum of all the individual dipole moments in a given system. Value of the dipole moment is zero when two individual dipole moments cancel out each other.
