Question
Question: Which compound does not show tautomerism? A. \( C{H_3} - COC{H_2}COC{H_3} \) B. \( {C_6}{H_5} -...
Which compound does not show tautomerism?
A. CH3−COCH2COCH3
B. C6H5−CH=N−OH
C. 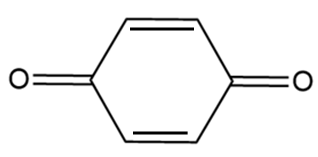
D.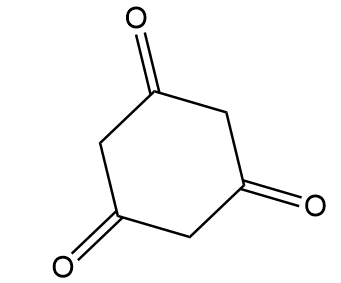
Solution
In order to find the number of tautomeric structures for a given compound, we first need to find the number of alpha hydrogen atoms i.e., number of hydrogen atoms bonded to the atom adjacent to the unsaturated group. That means the presence of alpha hydrogen atoms is a necessary condition for a compound to show tautomerism.
Complete Step By Step Answer:
Tautomerism: A phenomenon in which a single compound tends to exist in two or more than two interconvertible structures that generally differ in terms of the relative position of one atomic nucleus which is usually the hydrogen atom. During the reaction, an intramolecular proton transfer takes place and the structures in tautomerism exist in dynamic equilibrium.
Now, we know that for a compound to show tautomerism, there must be at least one alpha hydrogen present in the compound. So, the number of alpha hydrogen atoms in each given compound are as follows:
Compound-A:
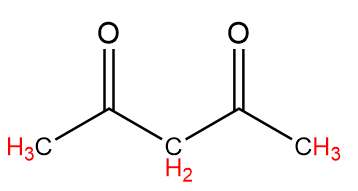
Number of alpha hydrogen atoms =8
Hence, this compound tends to show tautomerism.
Compound-B:
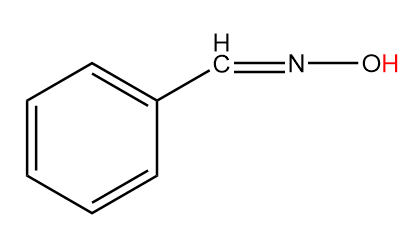
Number of alpha hydrogen atoms =1
Hence, this compound tends to show tautomerism.
Compound-C:
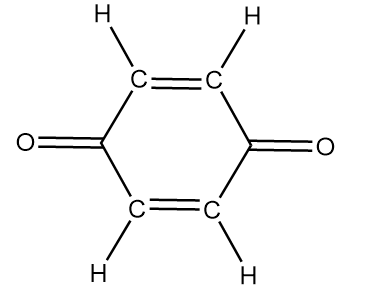
Although the compound consists of alpha hydrogens but if we draw the resulting structure, it is observed that a pair of cumulated double bonds are formed as follows:
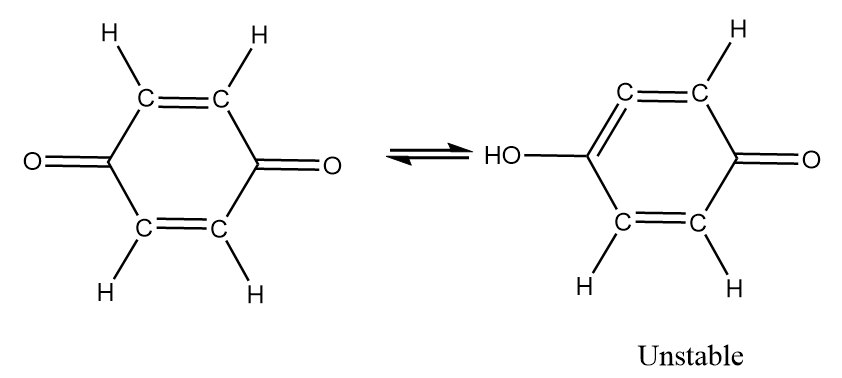
In the structure, the cumulated double bonds tend to be unstable because the carbon favours the 180o bond angle, which makes the ring highly strained and unstable. Hence, the given compound i.e., 1,4-benzoquinone does not show tautomerism.
Compound-D:
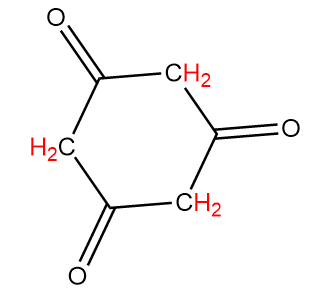
Number of alpha hydrogen atoms per carbonyl group =4
Hence, this compound tends to show tautomerism.
Therefore, we can conclude that compound-C does not show tautomerism.
So, option (C) is the correct answer.
Note:
Remember that the presence of alpha hydrogen in the compound is the necessary but not sufficient condition to check tautomerism. It is important to note that the phenomenon of tautomerism of ketone works better when the alpha hydrogen is present on a saturated carbon atom.
