Question
Question: Which compound does not have tautomerism? A.\[C{H_3} - COC{H_2}COC{H_3}\] B.\[{C_6}{H_5} - CH...
Which compound does not have tautomerism?
A.CH3−COCH2COCH3
B.C6H5−CH=N−OH
C.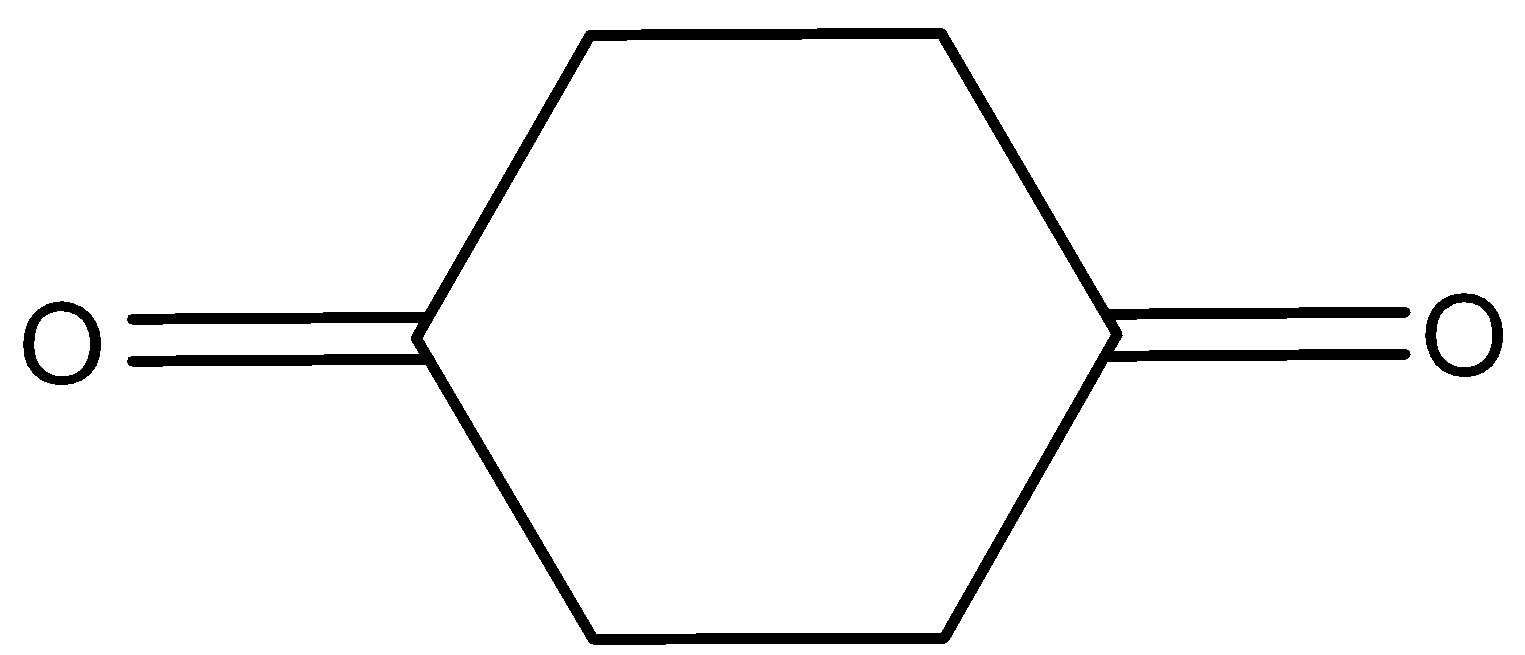
D.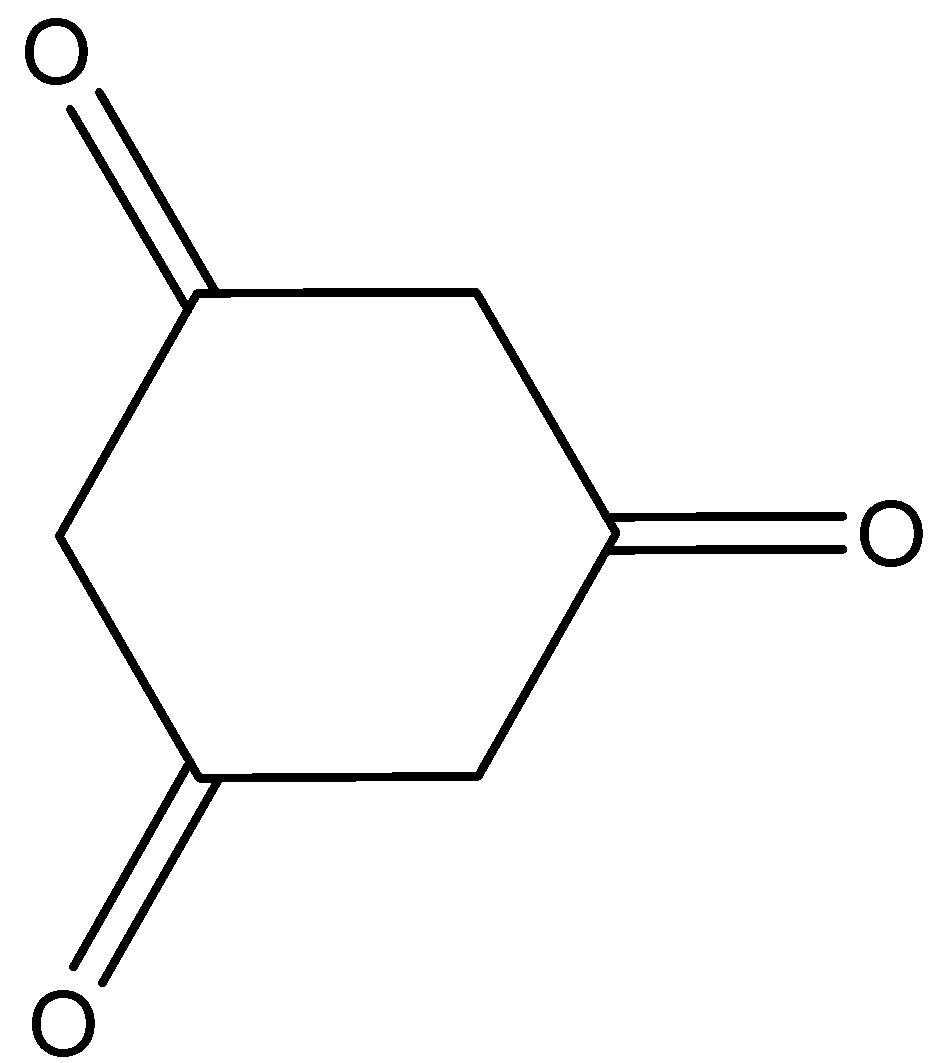
Solution
The compounds with different positions of protons and electrons are known as tautomers. And these are the constitutional isomers. Here, the carbon Skeleton of the compound will not change. A reaction that takes place by the simple transfer of protons in an intramolecular manner is known as tautomerism. And the isomer of phenol is an important example of tautomers.
Complete answer:
Acetyl acetone is a chemical compound having the formulaCH3−COCH2COCH3and it is classified as 1,3−diketone. It exhibits a tautomer in equilibrium condition and that is, CH3(O)CH=(OH)CH3. And this tautomer is interconverted very quickly. That is,
CH3−COCH2COCH3⇌CH3(O)CH=(OH)CH3
Hence, option (A) is incorrect.
The benzaldehyde oxime is an organic compound having the chemical formula, C6H5−CH=N−OH. And it will show the tautomerism and that is,
C6H5−CH=N−OH⇌C6H5−CH2−N=O
Hence, the option (B) is incorrect.
Here, the cyclohexane−1,4−dione will not show the tautomerism. Because here the alpha hydrogen is attached to the sp2hybridized carbon atom and it is very difficult to remove. Therefore, it does not show tautomerism.
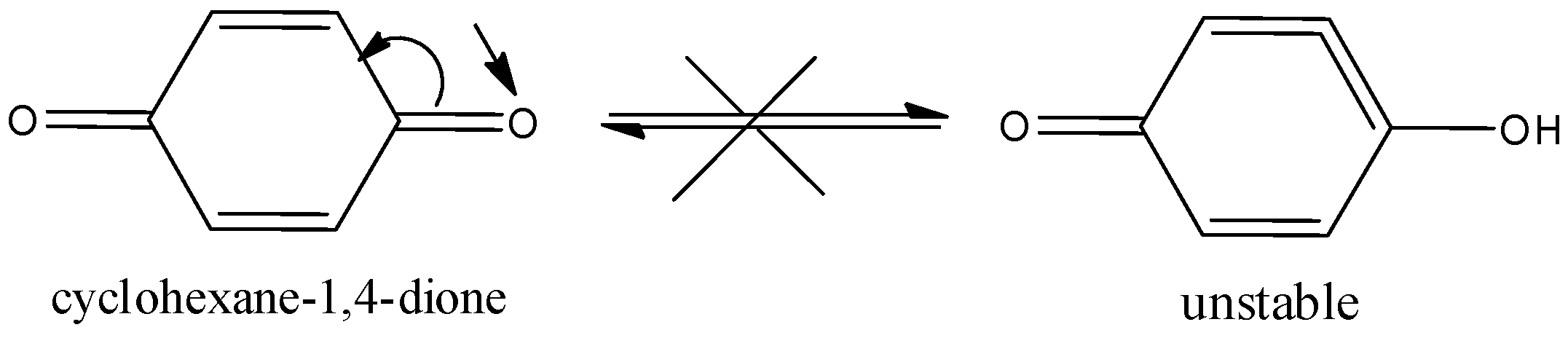
Hence, option (C) is correct.
Here, cyclohexane−1,3,5−trione shows the tautomerism. And that is,
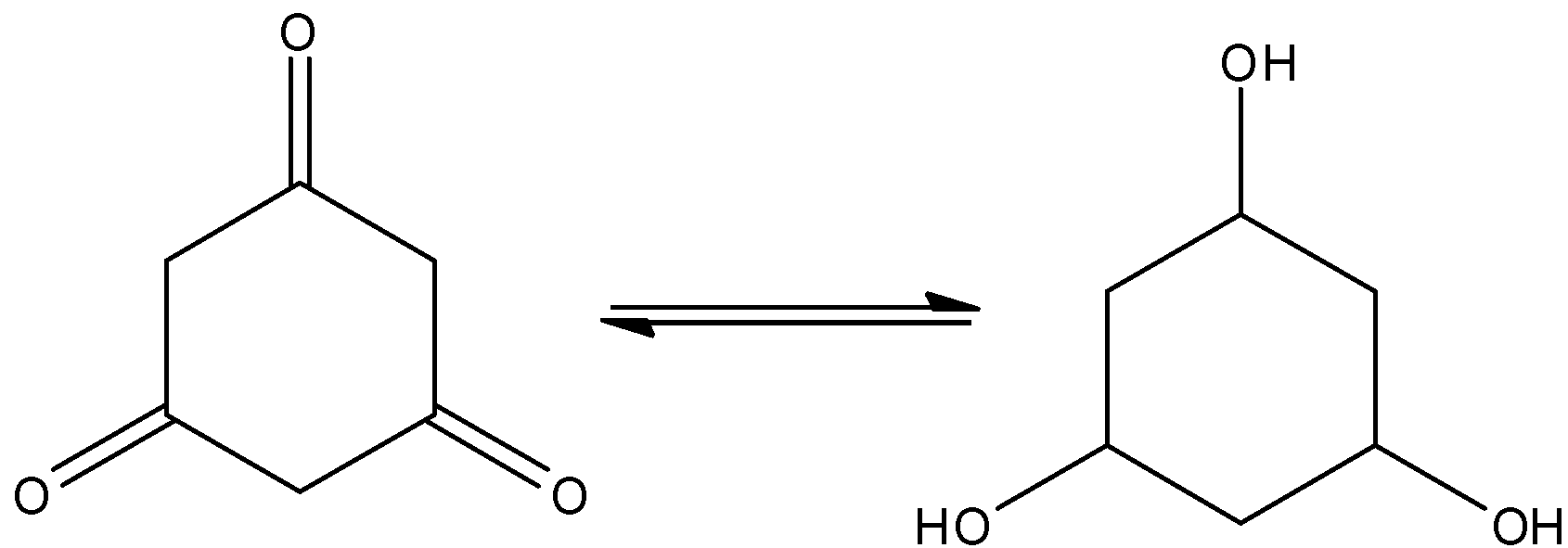
Hence, the option (D) is incorrect.
Hence, option (C) is correct.
Note:
If the compound does not contain the alpha hydrogen atom or the alpha hydrogen is attached to thesp2 hybridized carbon atom, then that compound will not show the tautomerism. Because the alpha hydrogen is very difficult to remove from the sp2hybridized carbon atom. For another example, in the case of benzaldehyde, which does not contain sp2hybridized carbon atoms. Therefore, it will not show tautomerism.
