Question
Question: Which compound does not give HVZ reaction? A. \({ CH }_{ 3 }-{ CH }_{ 2 }-{ CH }_{ 2 }-{ COOH }\) ...
Which compound does not give HVZ reaction?
A. CH3−CH2−CH2−COOH
B.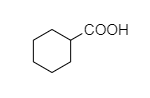
C. 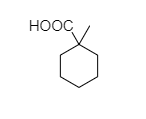
D. 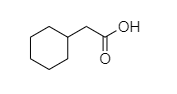
Solution
Hint: HVZ stands for Hell-Volhard-Zelinsky halogenation reaction. The HVZ reaction is halogenation of carboxylic acids at alpha carbon.
Complete answer:
This reaction is generally carried out by treating carboxylic acids with halogen in the presence of red phosphorus and then works up with water.
Those compounds which have alpha hydrogen show HVZ reaction while those which do not have alpha hydrogen do not show HVZ reaction.
A. In this CH3−CH2−CH2−COOH compound, alpha and beta hydrogen are present. That’s why butanoic acid shows a Hell-Volhard-Zelinsky halogenation reaction.
B. In cyclohexyl carboxylic acid, alpha hydrogen is present as shown below,
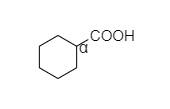
So, this compound also shows the Hell-Volhard-Zelinsky halogenation reaction.
C. In 1-methylcyclohexane-1-carboxylic acid, there is no alpha hydrogen present as the carbon is attached to 4 different bonds. So, this compound does not show the Hell-Volhard-Zelinsky halogenation reaction.
D. In 2-cyclohexyl acetic acid, alpha hydrogen is present as shown in the figure;
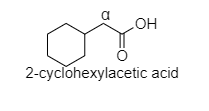
Hence, this compound shows the HVZ reaction.
The correct option is C.
Note: The possibility to make a mistake is that this reaction is specific for the replacement of alpha-hydrogens and not beta- hydrogens.
