Question
Question: Which compound does not give Cannizzaro reaction: A) Acetaldehyde B) Benzaldehyde C) Formaldeh...
Which compound does not give Cannizzaro reaction:
A) Acetaldehyde
B) Benzaldehyde
C) Formaldehyde
D) None of the above
Solution
In Cannizzaro reaction two molecules of aldehyde will undergo a redox reaction and will form a primary alcohol and a carboxylic acid by the use of a hydroxide base. For this reaction to take place there should be no α− Hydrogen present in the molecule. α− hydrogen means a hydrogen which is bonded with an α− carbon and α− carbon is the first carbon attached to the functional group.
Complete step-by-step solution:
To see which of the following compound does not contain α− hydrogen we will write the structural formula of the following:
Acetaldehyde =CH3−CHO
Benzaldehyde =C6H5−CHO
Formaldehyde =H−CHO
Here we see that only Acetaldehyde contains an α− hydrogen so it will not undergo the Cannizzaro reaction.
Here we will see how the reaction takes place:
2RCHO+NaOH→ROH+RCOOH
Where R= aryl or alkyl group which does not contain an α− hydrogen.
Now we will see the mechanism of reaction:
In the first step, a nucleophile as a hydroxide ion (which will come from sodium hydroxide) will attack the carbonyl group of the aldehyde.
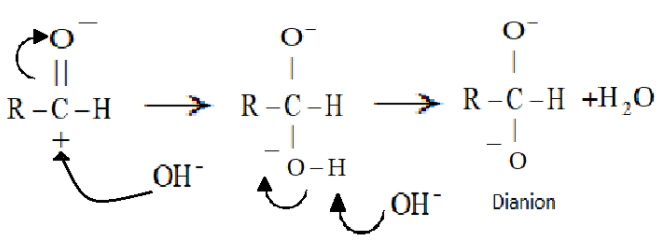
Now in the second step the resulting intermediate formed i.e. the dianion will function as a hydride reducing agent. It will release a hydride anion due to its unstable nature and will attack the carbon of another aldehyde molecule. A carboxylate anion and an alkoxide anion will be formed.
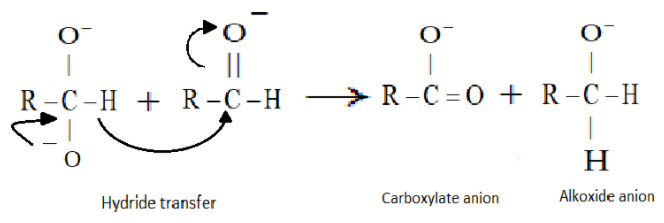
In the last step water molecules will offer a proton to the alkoxide anion and which will give rise to the alcohol. The reaction will proceed as the alkoxide anion is more basic than water. Carboxylate anion will give rise to the carboxylic acid by the use of an acid workup.
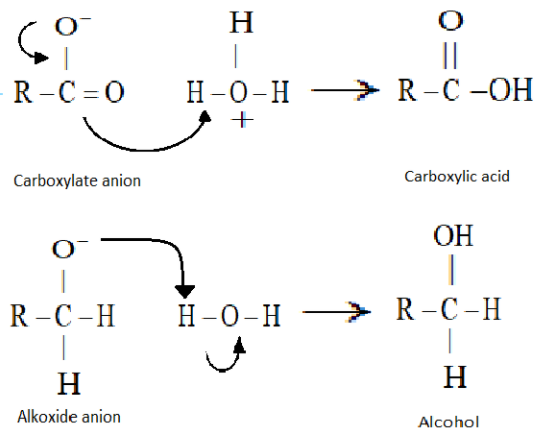
Hence an alcohol and carboxylic acid will be used.
Thus, the correct answer is Option A.
Note: This reaction is also known as a disproportionate reaction. An acid workup is used for the carboxylate anion as it is less basic than water and will not be able to extract a proton from water. Here acetaldehyde will undergo aldol condensation instead of Cannizzaro reaction.
