Question
Question: Which cannot show geometrical isomerism? (A)  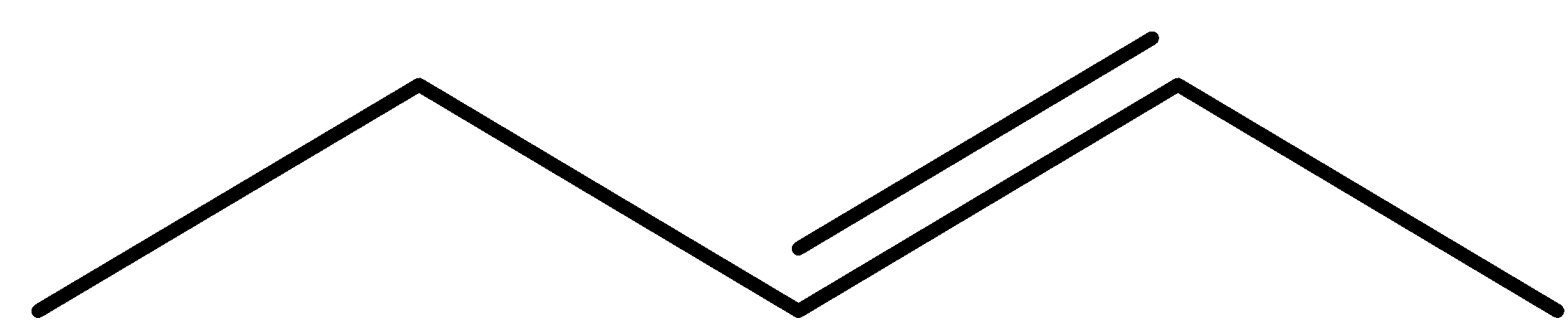
(B) 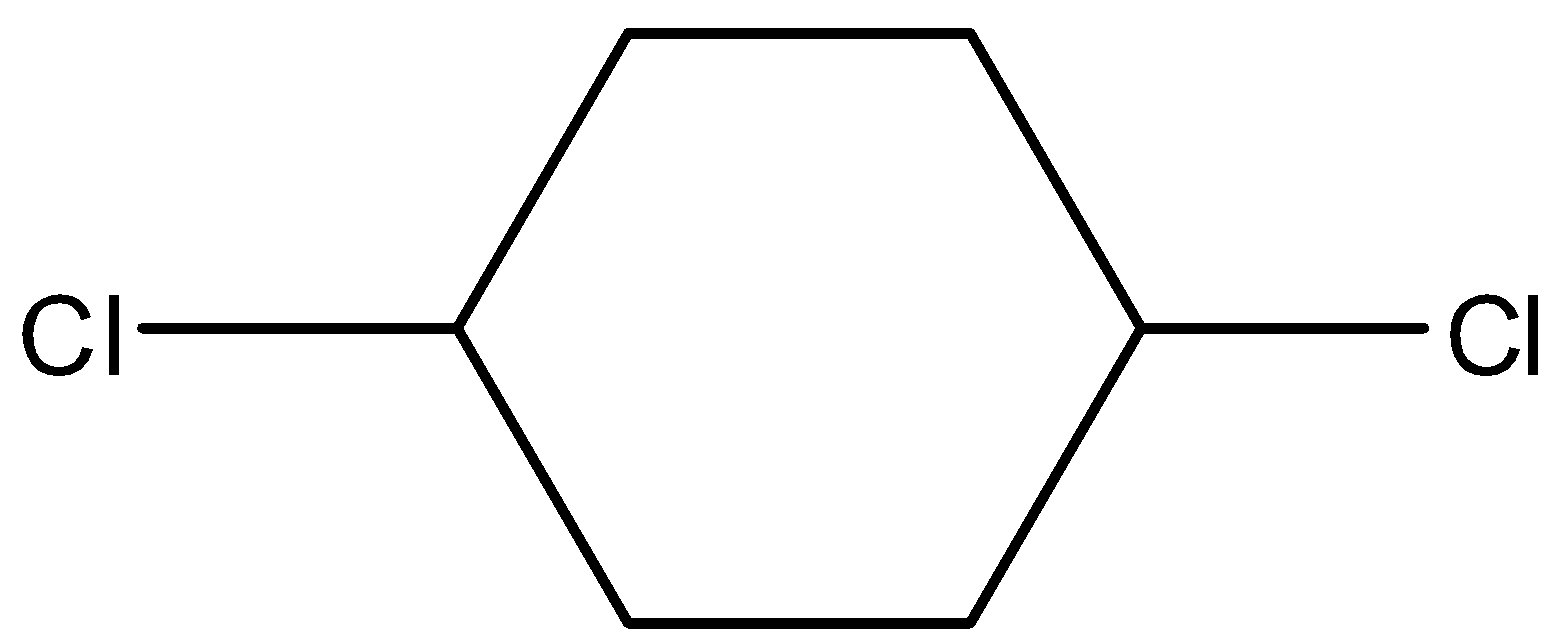
(C) 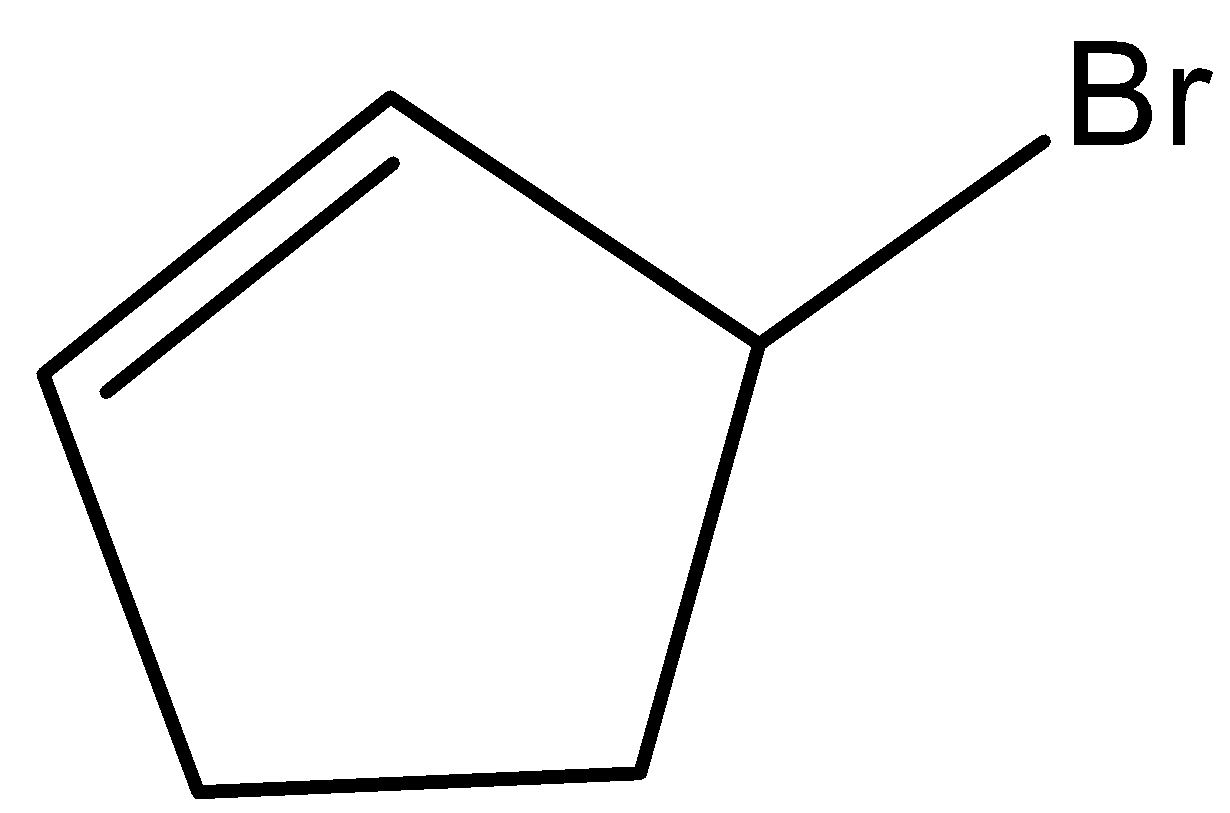
(D) 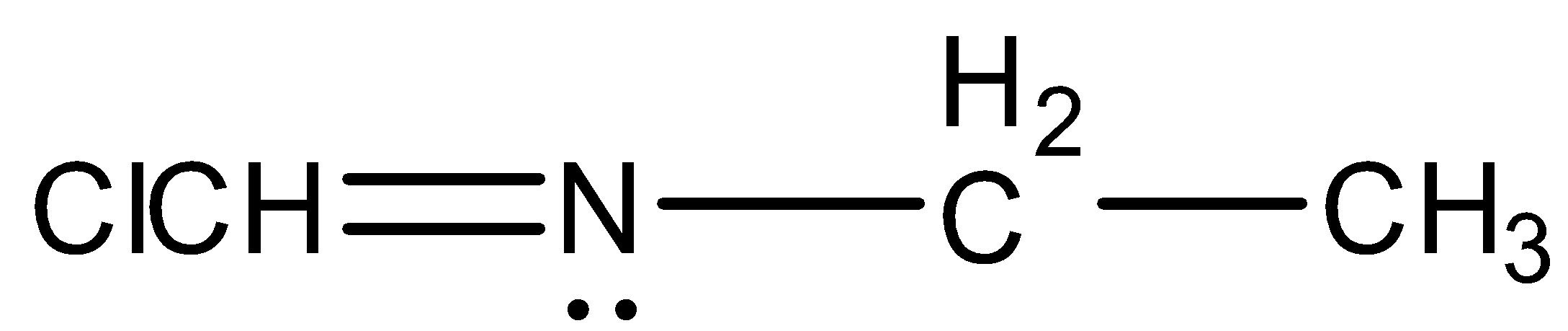
Solution
Hint Geometrical isomerism is possible in a molecule where there is a presence of a restricted rotation system (RRS). Geometrical is also called as Cis and trans isomerism also. The molecules which have double bonds generally show geometrical isomerism.
Complete step by step answer:
- In the question it is given that which molecule does not show the geometrical isomerism among the given options.
- Coming to the given options, option A.
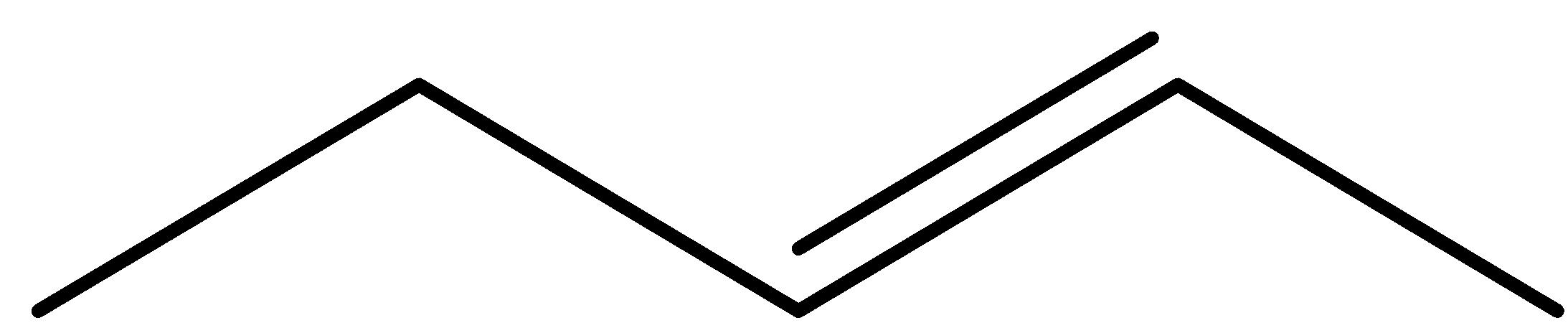
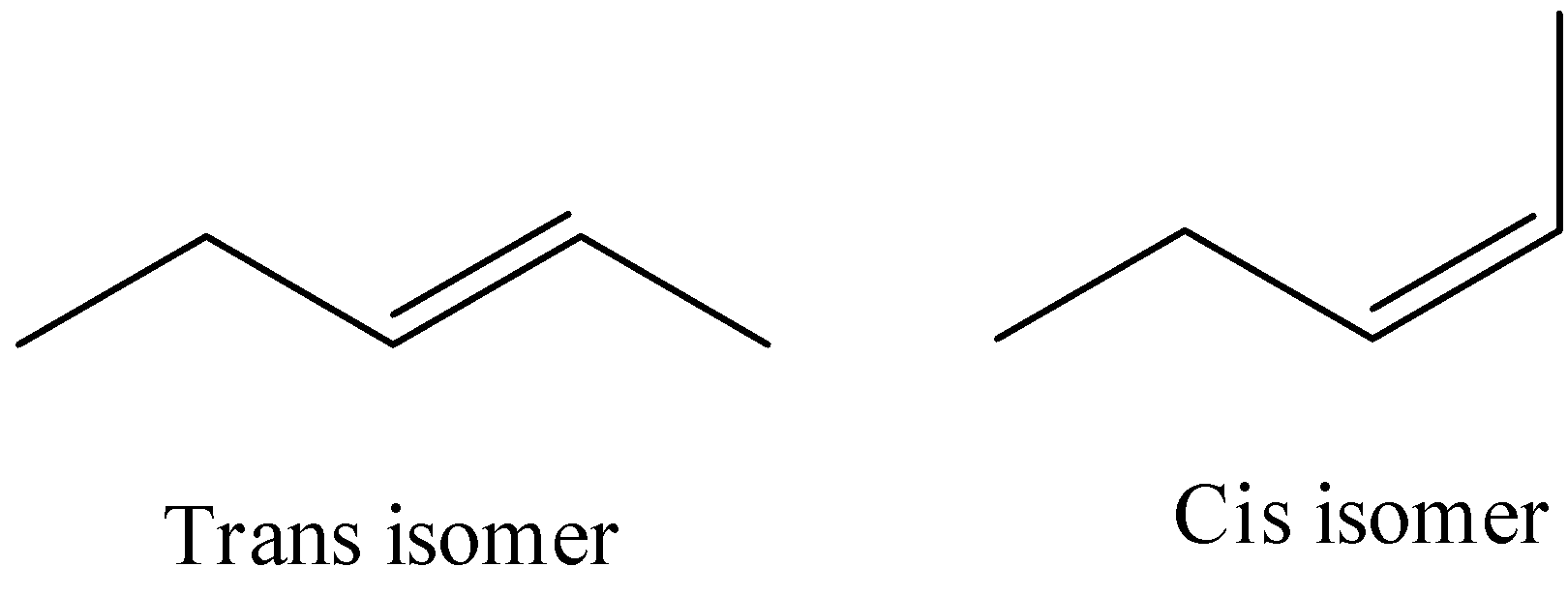
- The molecule in the option A is pent-2-ene, it has a restricted rotation system due to the presence of a double bond and it shows the geometrical isomerism and it is as follows.
- Coming to option B,
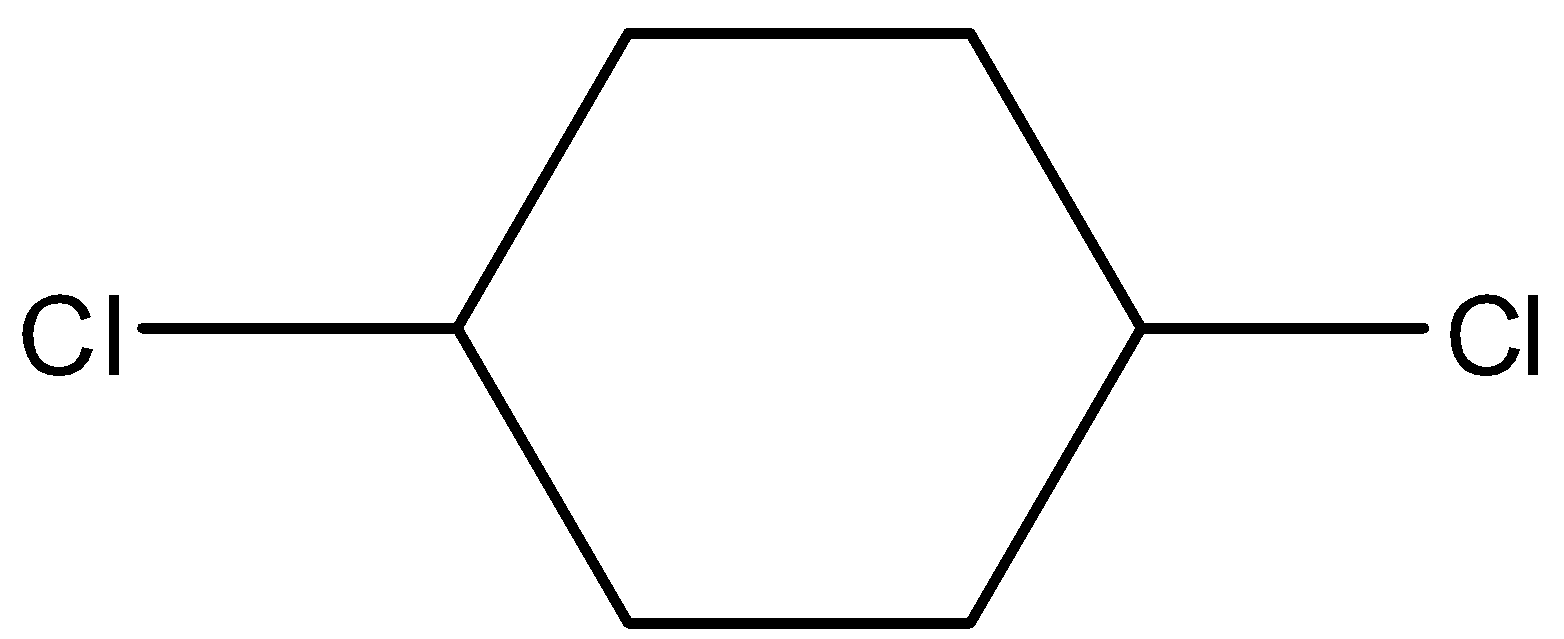
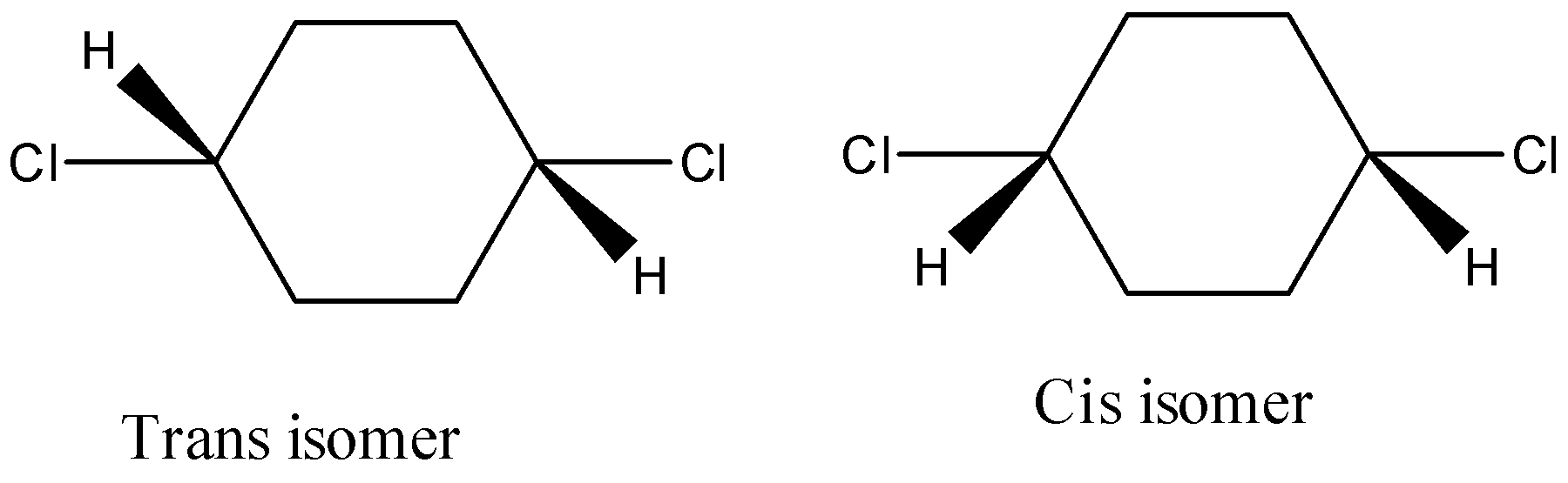
- It also has a restricted rotation system due to the presence of a cyclic ring and shows the geometrical isomerism and it is as follows.
- Coming to option C,
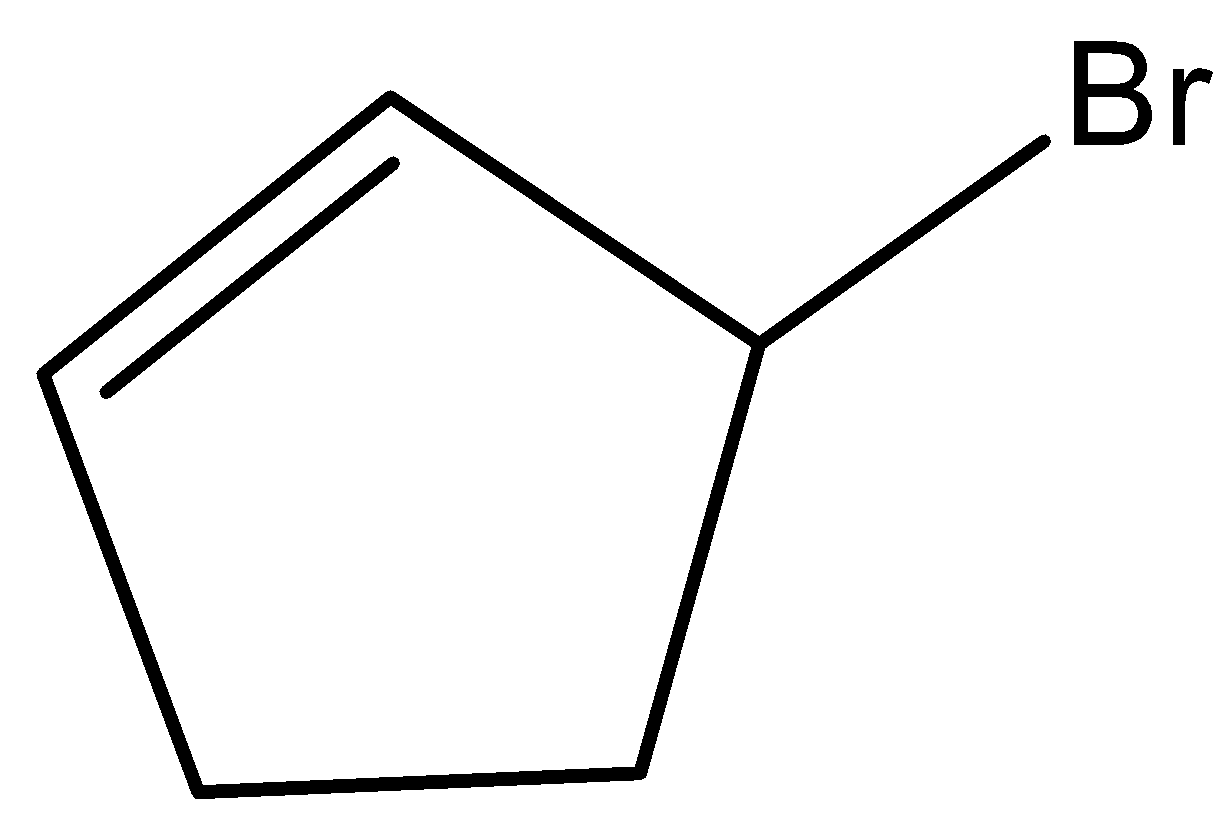
- This molecule won’t show the geometrical isomerism because the two hydrogens which are present on the carbons which have double bonds have the common end in the ring. Due to this reason option C won’t show geometrical isomerism.
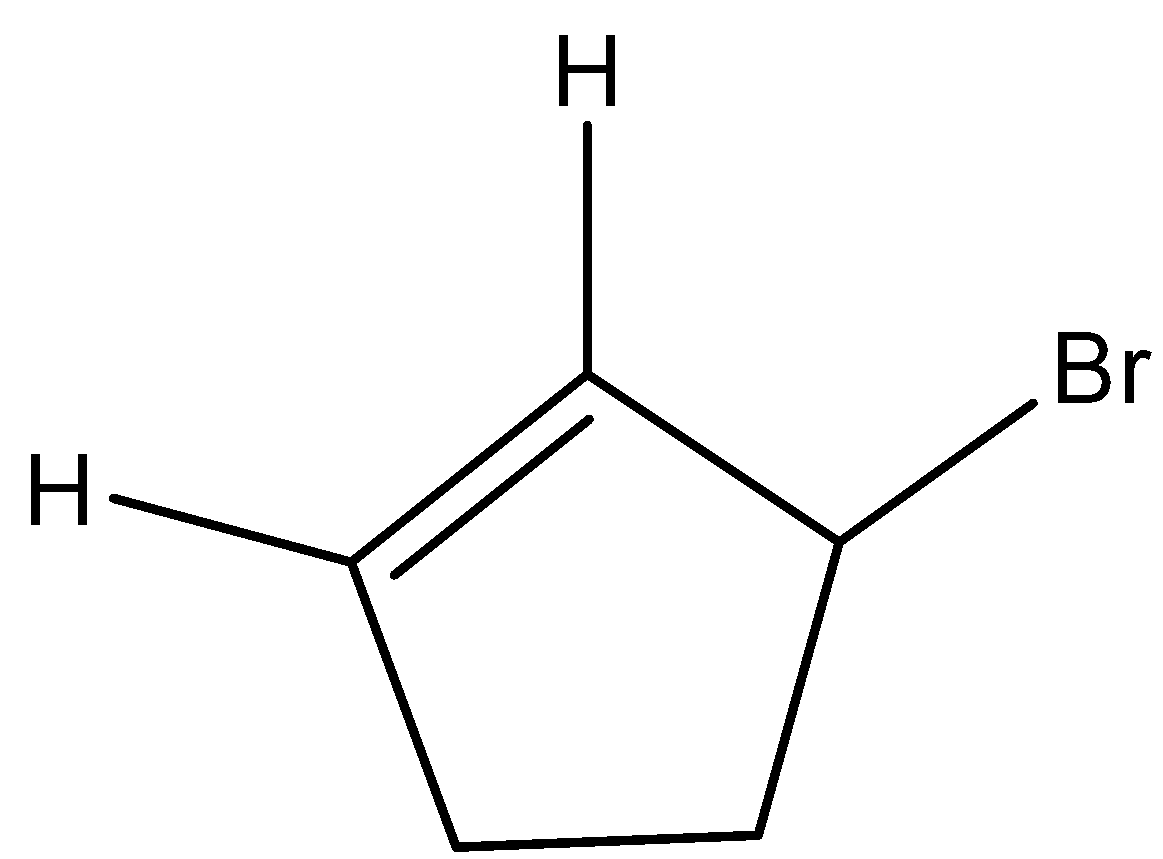
- Coming to option D,
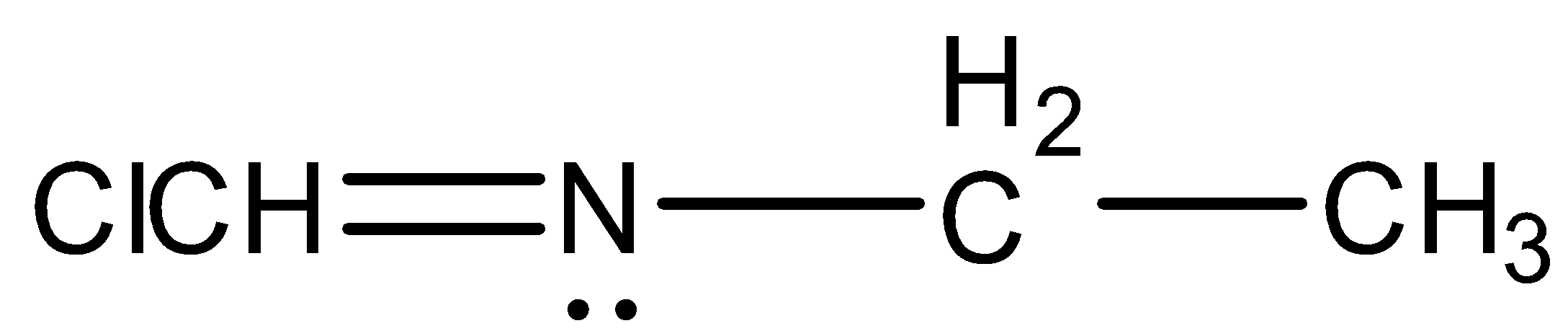
- The option D will show the geometrical isomerism due to the following forms of existence.

- Therefore the molecule which will not show geometrical isomerism among the following options is C.
Note: To show geometrical isomerism the carbon atoms which are present on both sides of the double bond should have different ends rather than the common end. Geometrical isomerism is also called as cis-trans isomerism.
