Question
Question: Which branched chain isomer of the hydrocarbon with molecular mass 72 u gives only one isomer of mon...
Which branched chain isomer of the hydrocarbon with molecular mass 72 u gives only one isomer of mono substituted alkyl halide?
(A) Tertiary butyl chloride
(B) Neopentane
(C) Isohexane
(D) Neohexane
Solution
Mono substituted alkyl halide has only one halogen atom in its structure. Isomers are compounds which have the same molecular weights but have different properties.
Complete step by step solution:
Isomers are the compounds which have the same molecular weights but have different properties. We will see the molecular structures of all the given molecules and then decide which one of them will have only one isomer of mono substituted alkyl halide.
A) Tertiary butyl chloride
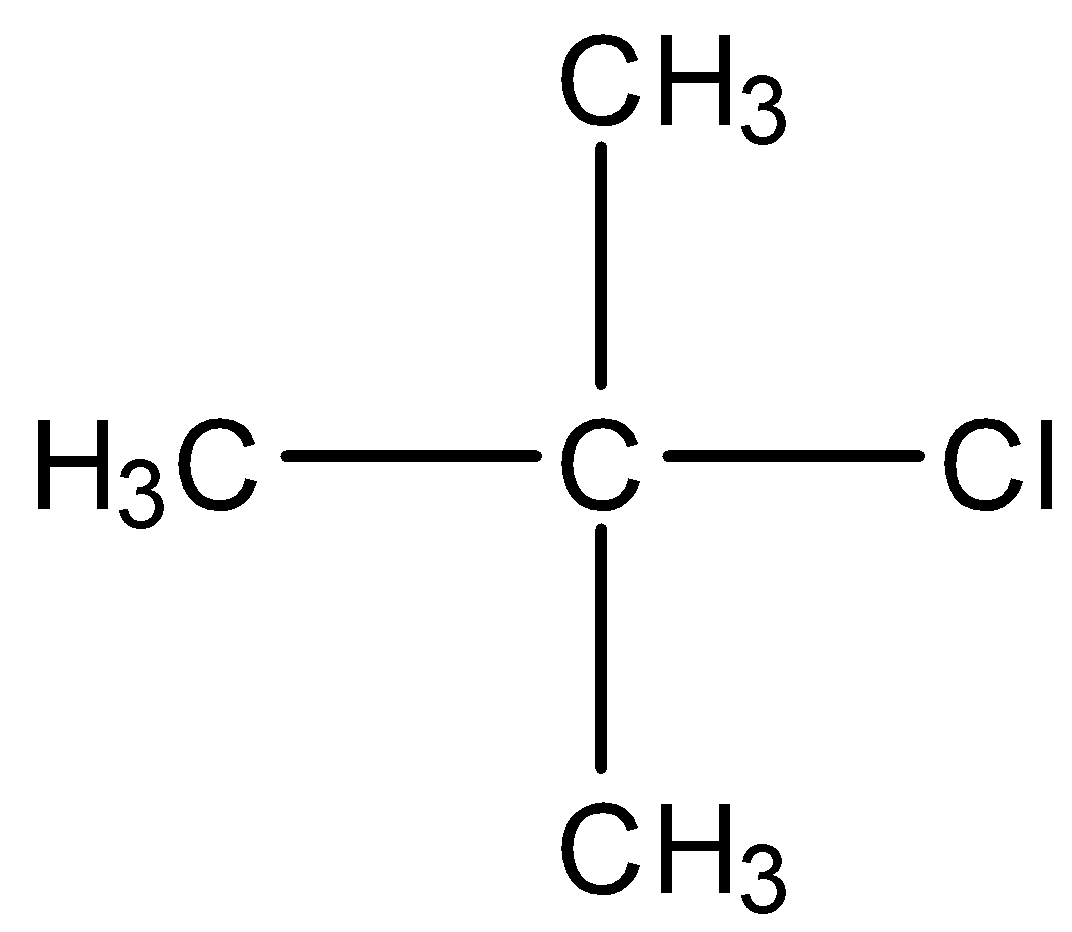
We can see that this molecule is already a mono substituted alkyl halide. Now, if we swap the positions of Cl and any of the H atoms. Then the new compound formed will give two isomers of the alkyl halide. So, this is not the correct answer.
B) Neopentane
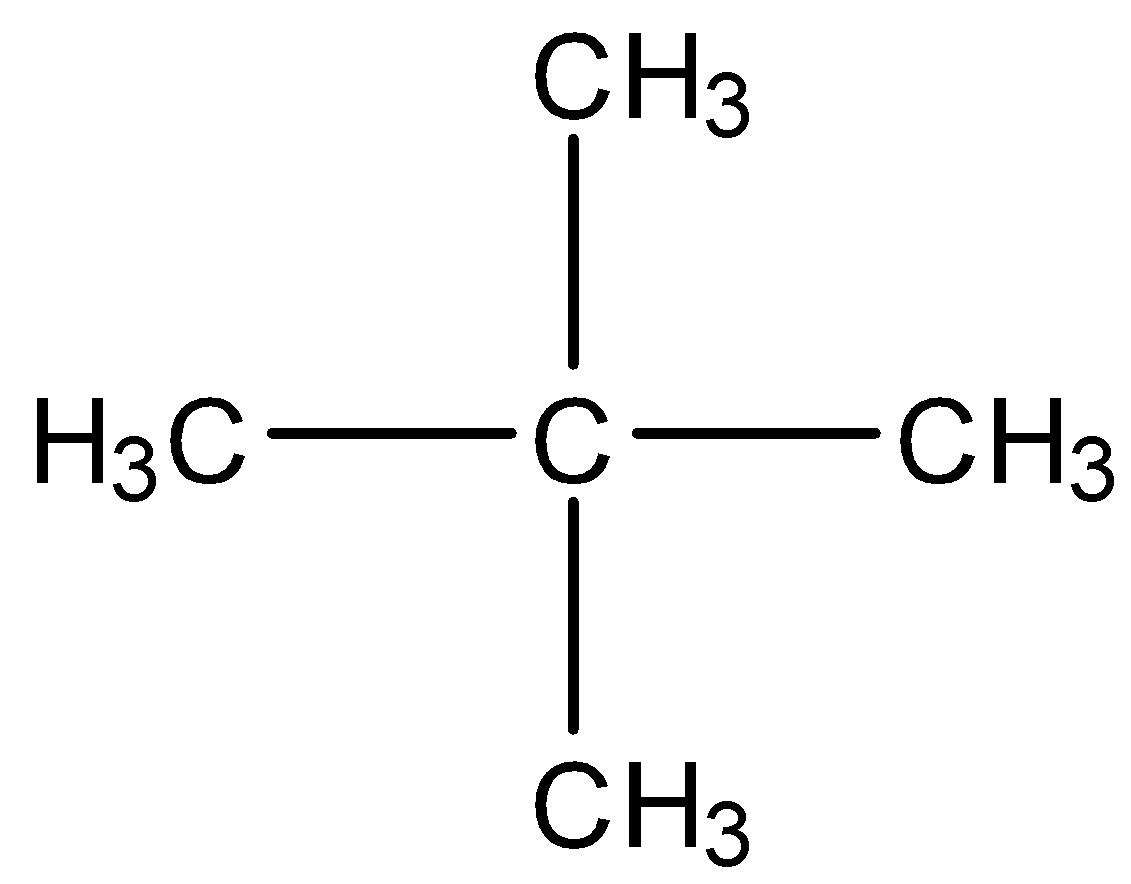
We can see that if we substitute any of the H atoms, then the mono substituted alkyl halide formed will have only one isomer because there are the same four methyl groups present at the carbon atom. Also neopentane has an atomic weight of 72 u. So, this is the correct answer.
C) Isohexane
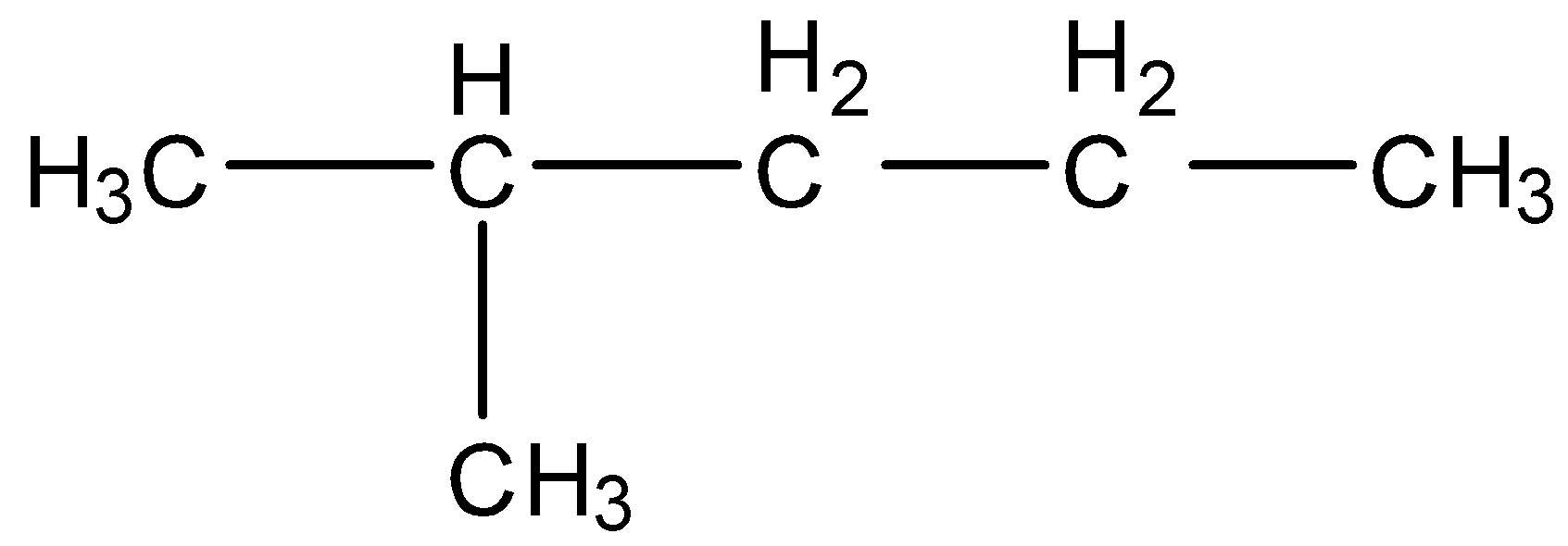
Here, if we substitute any H atom with a halogen atom, then there will be formation of more than one isomers. So, this is also not the correct answer.
D) Neohexane
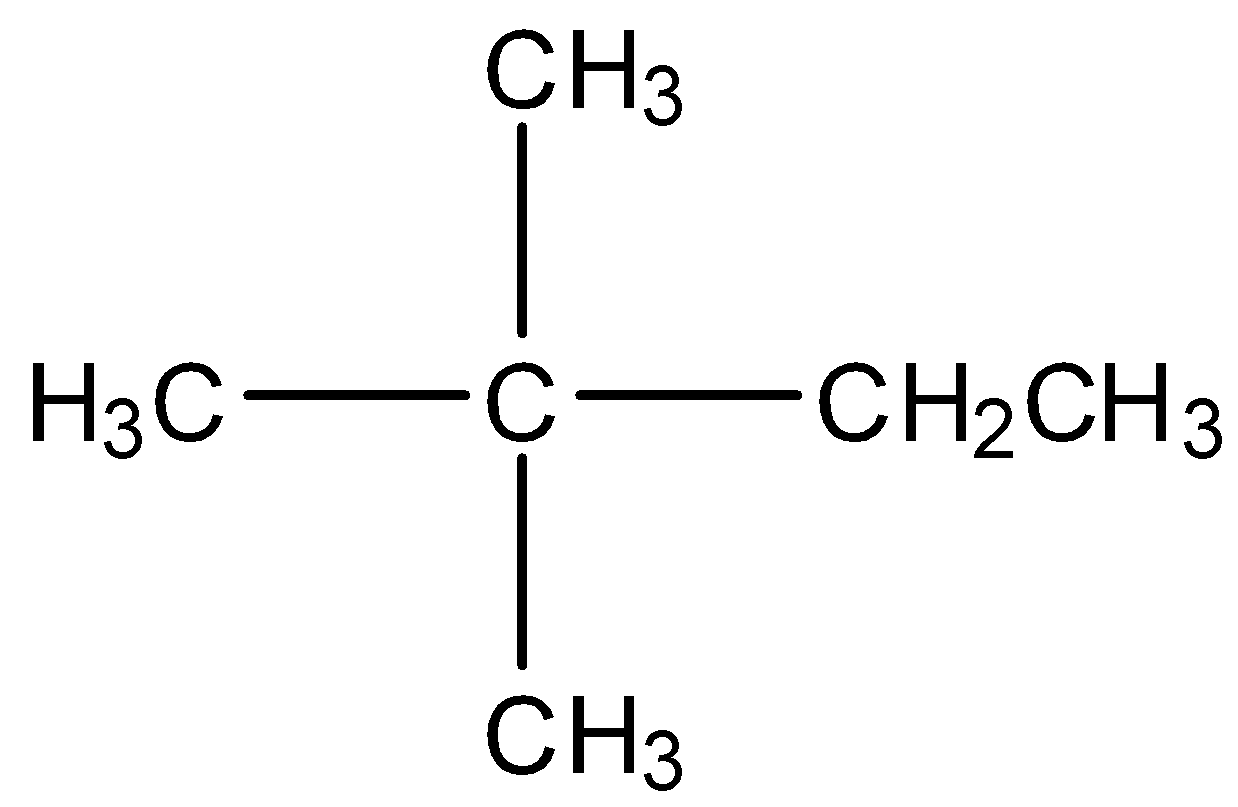
We can clearly see that here one carbon is extra than neopentane. So, the mass of this compound will be more than 72 u. So, this is also a wrong answer.
Thus, the correct answer is (B).
Note: Remember that whenever neo- prefix is used, then it shows the presence of a quaternary carbon. Same way, iso- prefix shows that there is a substitution of methyl group at the second last carbon atom.
