Question
Question: Which are true statements among the following? 1.\[P{H_5}\] and \[BiC{l_5}\] do not exist 2.P, d...
Which are true statements among the following?
1.PH5 and BiCl5 do not exist
2.P, d bonds are present in SO2
3.Electrons travel at the speed of light
4.SeF4 and CH4 have the same shape
5.I3+ has bent geometry
A.1,3
B.1,2,5
C.1,3,5
D.1,2,4
Solution
In order to solve this question, we must individually solve all the statements to determine the incorrect ones from them. Then in the end, we can group all these incorrect statements together and choose the corresponding option
Complete Step-by-Step Answer:
1. PH5 and BiCl5 do not exist:
PH5 as a compound, ceases to exist because p orbitals of phosphorus interact with s orbitals of hydrogen. A bond formed in this hybridized state is not stable. Hence it does not exist. A similar situation happens with BiCl5 . The +5-oxidation state of BiCl5 has a very low stability in comparison to its +3-oxidation state. Hence, even BiCl5 does not exist.
Hence, this statement is true.
2.P, d bonds are present in SO2 :
To answer this, it is better if we draw a Lewis Structure of SO2 .
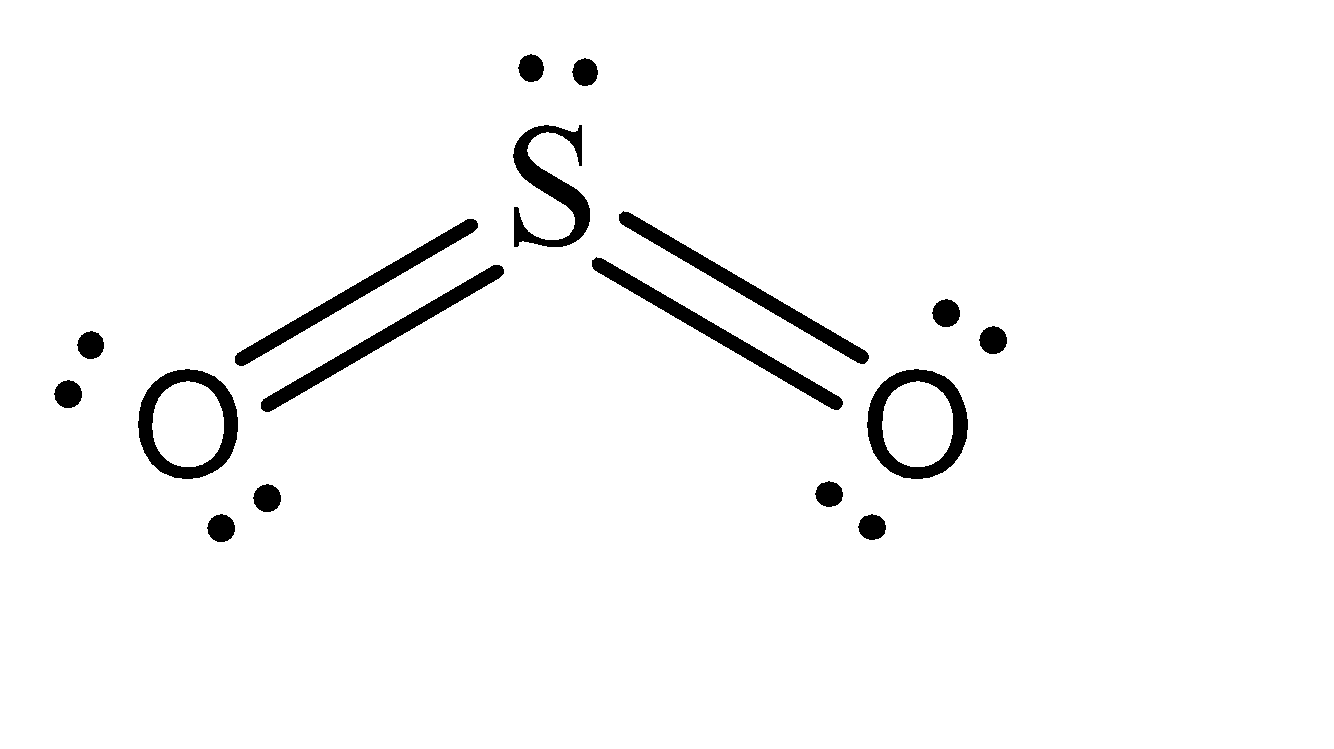
As we can see, 2 pi bonds are formed in the case of SO2 . Now the nature of these bonds can be determined by understanding the hybridization. The unpaired electrons of sulphur reside in the 3p and 3d hybridized orbitals, while the pi bonds are formed by interacting with oxygen’s 2p orbitals. Hence, the two pi bonds formed consist of one p−p pi bond and one p−d pi bond.
Hence, this statement is true
3.Electrons travel at the speed of light:
No entity which has a mass can travel at the speed of light. Electrons have a mass. Hence, it cannot travel at the speed of light.
Hence, this statement is false.
4. SeF4 and CH4 have the same shape:
To understand this statement, let us draw the structures of the two compounds.
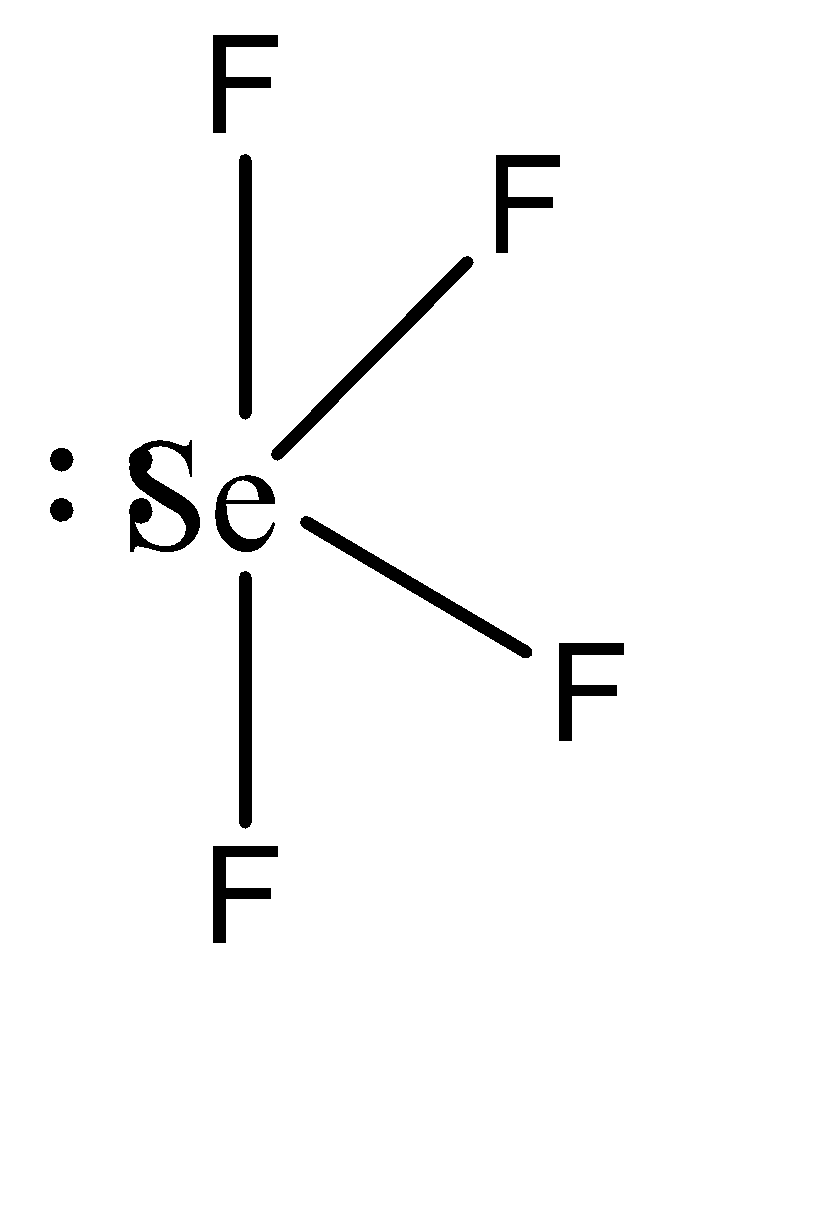
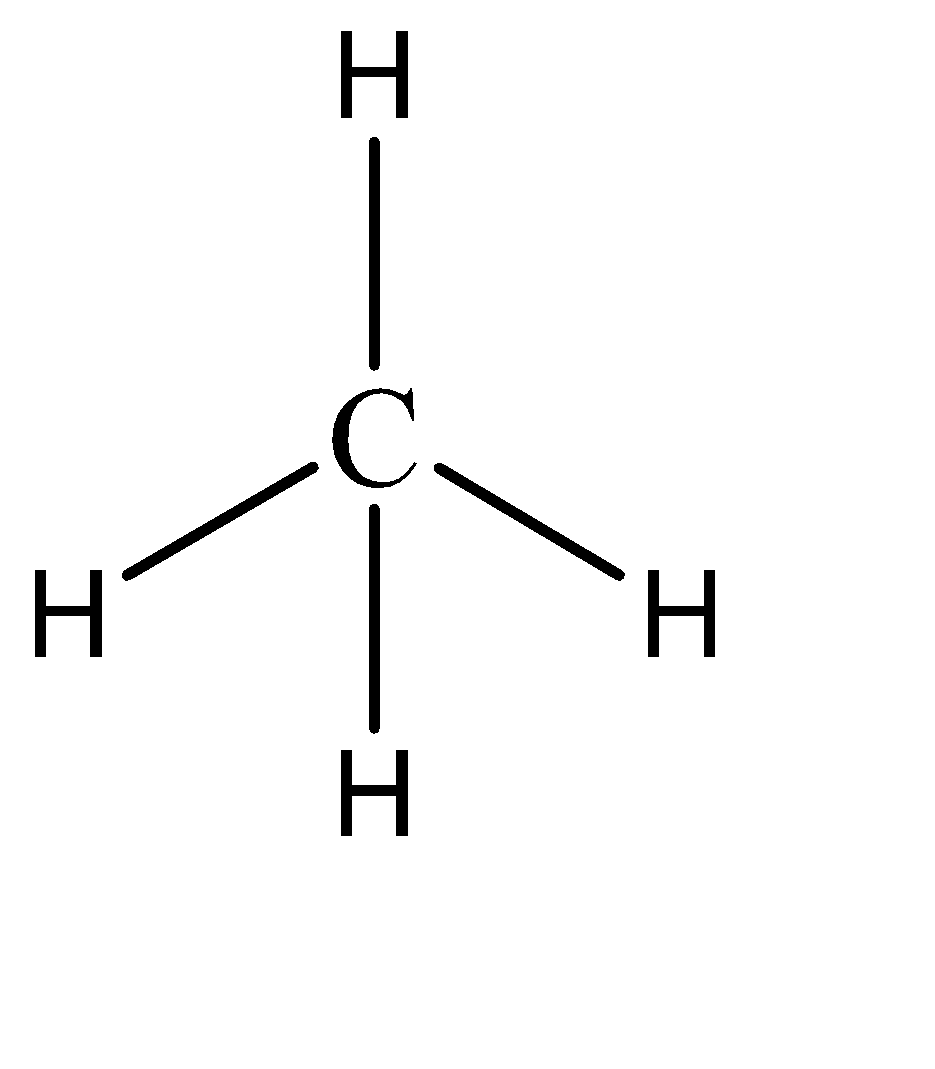
We can observe that SeF4 has a seesaw shape while CH4 has a tetrahedral shape. In SeF4 , the repulsion caused by the lone pair forces the other atoms to move away from it, resulting in the seesaw shape. Similarly, equivalent electron repulsion from all the hydrogen atoms in CH4 causes them to form a uniform tetrahedral geometry.
Hence, the given statement is false.
5. I3+ has bent geometry:
This statement is true because I3+ does have a bent geometry, because of its trigonal bi-pyramidal structure
Hence, statements 1, 2 and 5 are correct
Hence, Option B is the correct option.
Note: While solving for the last statement, students may often think about the structure of I3+ as a linear structure while drawing the Lewis Structure. But in reality, due to the presence of lone pairs on all three iodine atoms, it forms a trigonal bipyramidal structure.
