Question
Question: Which are the monomers of Buna-N? A. Buta-1,3-diene and prop-1-ene-1-nitrile B. Buta-1,3-diene a...
Which are the monomers of Buna-N?
A. Buta-1,3-diene and prop-1-ene-1-nitrile
B. Buta-1,3-diene and acrylonitrile
C. Buta-1,3-diene and prop-2-ene-1-nitrile
D. Buta-1,2-diene and prop-1-ene-1-nitrile
Solution
Hint: It is known that Buna-S has Buta-1,3-diene and Styrene as its monomer. The IUPAC name of styrene is ethenylbenzene and both Buna-S and Buna-N are copolymer.
Complete Step by Step Solution:
Try to recall that monomer is the smallest structural unit of a polymer. It is known that Buna-N is a co-polymer which is prepared by the addition polymerization method of monomers Buta-1,3-diene and acrylonitrile. So, in order to find out which option is correct, lets inspect all the above given options.
We should know that prop-1-ene-1-nitrile is the IUPAC name of acrylonitrile and Buta-1,3-diene and acrylonitrile are the monomers of Buna-N. So, option A is the correct answer.
Now, we already know that Buta-1,3-diene and acrylonitrile are the monomers of Buna-N. So, option B is also the correct answer.
The structure of Buta-1,3-diene is:
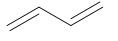
And, the structure of acrylonitrile or prop-1-ene-1-nitrile is:
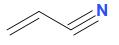
Therefore, it is concluded that both option a and b are correct.
Additional Information:
Copolymer is defined as “a polymer which is formed when two or more different types of polymer are linked with each other in the same polymer chain”. Example- Buna-S which is formed by the copolymerization of monomers recall that monomer is the smallest structural unit of a polymer and Styrene.
Additional polymers are defined as “Those polymers which are formed by simple linking of monomers without formation of any by-product”. Example-Polyethylene which is formed by addition polymerization method having ethylene as its monomer.
Note: Addition polymerization is also called chain growth polymerization.
Buna-N is also known as NBR which stands for Nitrile-Butadiene-Rubber.
