Question
Question: Which are position isomers? A.  and
and 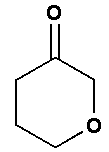
B.
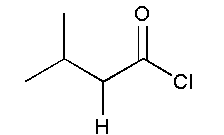 and
and 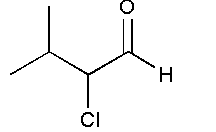
C.
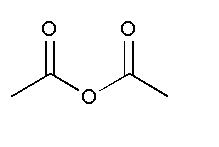 and
and 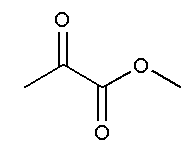
D.
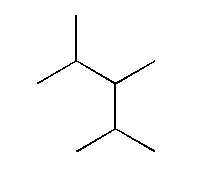 and
and 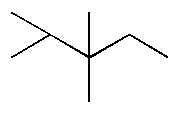
Solution
Position isomers are the structural isomerism or constitutional isomers which have the same molecular formula but the positions of the functional groups are different in both the compounds.
Complete step by step answer:
Isomers are defined structures which possess similar molecular formulas but differ in the arrangement of atoms.
The isomers are divided into conformational isomers and structural isomers.
The conformational isomers are defined as the conformers which differ from each other on the basis of rotation around the single bond.
Structural isomers are defined as the isomers which have the same molecular formula but differ in their structural formula. Structural isomers are also known as constitutional isomers.
The position isomers are the constitutional isomers which have the same chemical formula or molecular formula but the arrangement of the functional group is different in different structures.
In option A, no functional group is present. Therefore, it will not show position isomer.
In option B, no functional group is present. Therefore, it will not show position isomer.
In option C, no functional group is present. Therefore, it will not show position isomer.
In option D, methyl is the non-polar functional group which is placed at different positions in both the compounds. The first compound is 2, 3, 4 Tri methyl pentane and the other compound is 2, 3, 3 Tri methyl pentane.
Therefore, the correct option is D.
Note: Don’t get confused over constitutional isomerism, skeletal isomerism and position isomerism as the skeletal isomerism and position isomerism come under constitutional isomerism. The skeletal isomerism deals with the change in the bonding and atom in the structures.
