Question
Question: Which are pair of Resonating structures?...
Which are pair of Resonating structures?
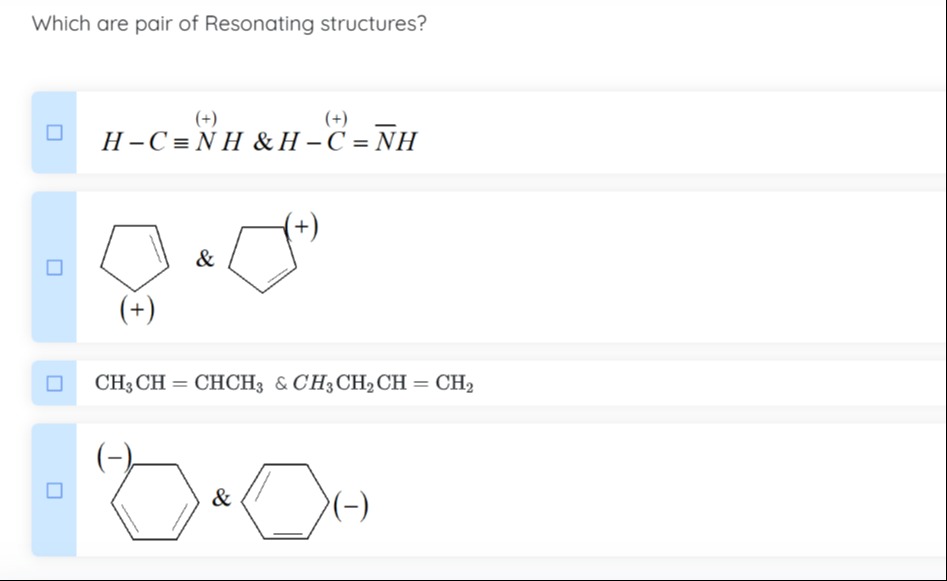
H−C≡N(+)H & H−C(+)=NH
CH3CH=CHCH3 & CH3CH2CH=CH2
The two species, CH3CH=CHCH3 (2-butene) and CH3CH2CH=CH2 (1-butene), are structural isomers. They have different atomic connectivity and are different molecules, not resonance structures of the same molecule.
The structures show a 6-membered ring with alternating double and single bonds and a negative charge on different carbon atoms. This represents the delocalization of a negative charge in a conjugated system, such as a phenyl carbanion (C6H5−). The atomic connectivity is the same, and the electrons (negative charge and pi bonds) are redistributed, making them resonance structures.
Options 1 and 4
Solution
Resonating structures are Lewis structures of a molecule or ion that differ only in the arrangement of electrons, particularly pi electrons and lone pairs. The connectivity of atoms must remain the same.
Option 1: H−C≡N+−H and H−C+=N−H (with a lone pair on N). These have the same atomic connectivity and differ in electron distribution.
Option 4: A 6-membered ring with alternating double bonds and a negative charge on different carbons. This shows delocalization of charge in a conjugated system.
Options 2 and 3 represent structural isomers, not resonance structures, as they have different atomic connectivity.
