Question
Question: Which amongst the following metal carbonyls are inner orbital complexes with diamagnetic property? ...
Which amongst the following metal carbonyls are inner orbital complexes with diamagnetic property?
(i) Ni(CO)4
(ii) Fe(CO)5
(iii) V(CO)6
(iv) Cr(CO)6
Select the correct answer from the codes given below:
(A) I and II only
(B) II, III and IV only
(C) II and IV only
(D) I and II only
Solution
Hint : The coordination compounds in which the hybridization state of the central metal includes d orbitals from inner shell while s and p orbitals from outer shell are known as inner orbital complexes. These are also known as low spin complexes and observed only if the ligand bonded to the central metal atom is a strong field ligand.
Complete Step By Step Answer:
To check the magnetic properties and the type of complexes, we need to write the electronic configuration for each central metal given in the compounds. The electronic configuration for given complexes is as follows:
Ni(CO)4 :
The ground state electronic configuration of nickel =3d84s2
As CO is the strong field ligand, so all the ten electrons paired up in d orbital leaving s and p orbitals vacant. Then, the 4s and 4p orbitals participate in the bonding with CO to give Ni(CO)4 . The hybridization state of nickel is sp3 and it has a tetrahedral structure.
The formation of bonds in Ni(CO)4 is explained as follows:
Electronic configuration of nickel at ground state:
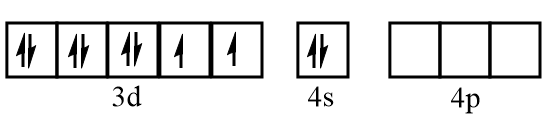
Electronic configuration of nickel when CO molecules approach toward it:
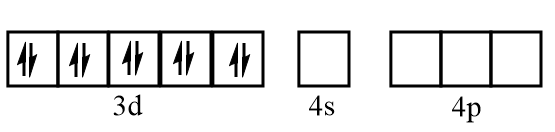
Electronic configuration of nickel after formation of Ni(CO)4 complex:
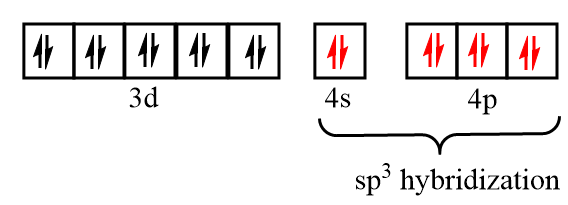
As there is no unpaired electron present in the orbitals, so Ni(CO)4 is diamagnetic in nature and because no inner d orbital participates in the hybridization of the complex, so it is not considered as an inner orbital complex.
Fe(CO)5 :
The ground state electronic configuration of iron =3d64s2
As CO is the strong field ligand, so all pairing of electrons takes place in the d-orbital due to which the hybridization state of the iron after formation of complex becomes dsp3 .
The formation of bonds in Fe(CO)5 is explained as follows:
Electronic configuration of iron at ground state:
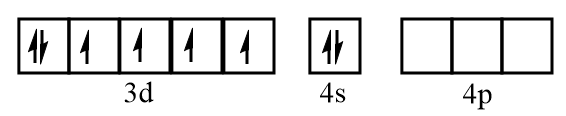
Electronic configuration of iron when CO molecules approach toward it:
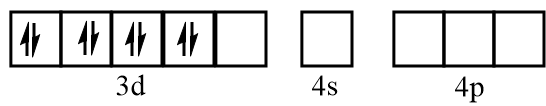
Electronic configuration of iron after formation of Fe(CO)5 complex:
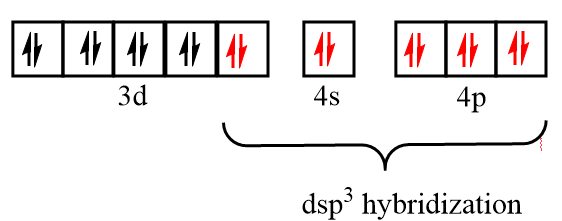
As there is no unpaired electron present in the orbitals, so Fe(CO)5 is diamagnetic in nature and due to participation of the inner d orbital in hybridization, it is known as the inner orbital complex.
V(CO)6 :
The ground state electronic configuration of vanadium =3d34s2
As CO is the strong field ligand, so all pairing of electrons takes place in the d-orbital due to which the hybridization state of the vanadium after formation of complex becomes d2sp3 .
The formation of bonds in V(CO)6 is explained as follows:
Electronic configuration of vanadium at ground state:
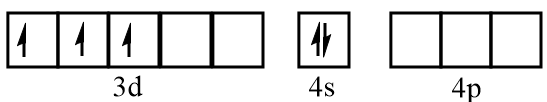
Electronic configuration of vanadium when CO molecules approach toward it:
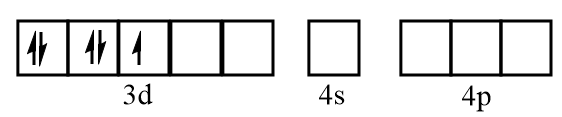
Electronic configuration of vanadium after formation of V(CO)6 complex:
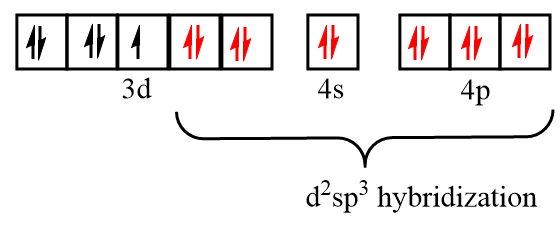
As there is one unpaired electron present in the orbitals, so V(CO)6 is paramagnetic in nature and due to participation of the inner d orbital in hybridization, it is known as the inner orbital complex.
Cr(CO)6 :
The ground state electronic configuration of chromium =3d54s1
As CO is the strong field ligand, so all pairing of electrons takes place in the d-orbital due to which the hybridization state of the chromium after formation of complex becomes d2sp3 .
The formation of bonds in Cr(CO)6 is explained as follows:
Electronic configuration of chromium at ground state:
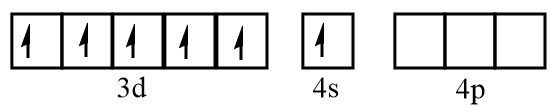
Electronic configuration of chromium when CO molecules approach toward it:
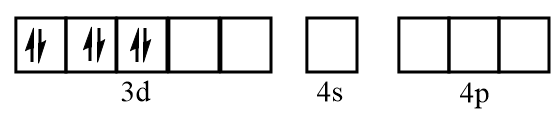
Electronic configuration of chromium after formation of Cr(CO)6 complex:
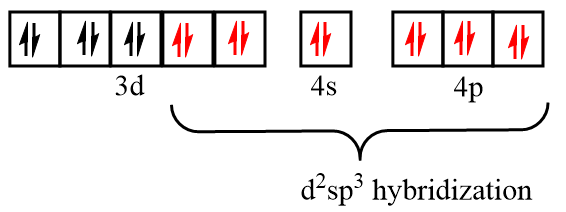
As there is no unpaired electron present in the orbitals, so Cr(CO)6 is diamagnetic in nature and due to participation of the inner d orbital in hybridization, it is known as the inner orbital complex.
Hence, only II and IV compounds are inner orbital complexes with diamagnetic properties. So, option (C) is the correct answer.
Note :
It is important to note that the compounds in which are bonded with weak field ligands and the outer d orbitals participate in the hybridization, that compound is known as outer orbital complex or high spin complex. Pairing of electrons does not take place in such complexes.
