Question
Question: Which amongst the following is the most stable carbocation? A. 
B. 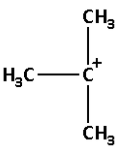
C. 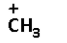
D. 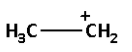
Solution
We know that carbocation is a positively charged carbon atom. The carbocation with the least partial positive charge is more stable. This is because the charge is distributed among neighbouring carbons. Higher the charge density on the carbon atom, more unstable is the carbocation.
Complete step by step solution:
->The effect regarding the transmission of unequal sharing of the bonding electrons through a chain of atoms in a molecule which leads to permanent dipole in a bond is known as the inductive effect.
->The methyl (−CH3) group pushes electron density towards the carbon having positive charge and shows an inductive effect. As the methyl group pushes electron density towards the carbon having positive charge the charge density on the carbocation decreases.
Thus, more the number of methyl groups, more stable is the carbocation.
->We are given four carbocations. Carbocation (A) has two methyl groups, carbocation (B) has three methyl groups, carbocation (C) has no methyl group but has three hydrogen atoms and carbocation (D) has one methyl group.
Thus, carbocation (B) has the highest number of methyl groups and so it is the most stable carbocation.
**Thus, the correct option is (B).
Note: **
When a carbon atom carrying a positive charge is attached to only one alkyl group it is known as a primary carbocation. When a carbon atom carrying a positive charge is attached to two other alkyl groups it is known as a secondary carbocation. When a carbon atom carrying a positive charge is attached to three other alkyl groups it is known as a tertiary carbocation. A tertiary carbocation is more stable than the secondary carbocation which is more stable than the primary carbocation.
