Question
Question: Which among the following is the correct IUPAC name? A. 1,1-Dimethylcyclohexan-3-ol B. \(4 - {\t...
Which among the following is the correct IUPAC name?
A. 1,1-Dimethylcyclohexan-3-ol
B. 4−methylbicyclo[3⋅2⋅0]heptane
C. Butane-1,2-dione
D. 4-ethyl-5-methylcyclohexene
Solution
In organic chemistry, IUPAC nomenclature is a method to name organic compounds which is recommended by the international union of pure and applied chemistry. Although a single compound can have more than one name, IUPAC set some rules to give a standard name to the compound.
Complete answer: IUPAC has set different rules for different types of organic compounds like rules are different for aliphatic compounds, aromatic compounds and bicyclic compounds. Let’s look at the structure of each given compound in the question and its name as per IUPAC rules.
Compound A ⇒ 1,1-Dimethylcyclohexan-3-ol
The given compound is structurally represented as follows:
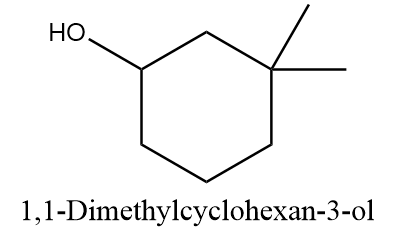
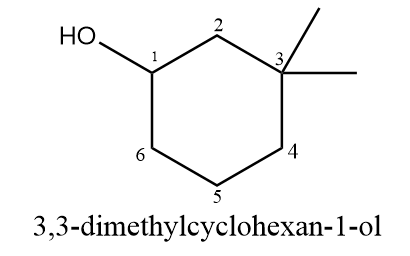
According to IUPAC, if the number of carbon atoms in the alkyl groups attached to the ring are less than the number of carbon atoms in the ring, then the ring is considered as the parent chain. If both hydroxyl group and alkyl group are substituted to the ring, then the priority is given to the hydroxyl group i.e., numbering will start from the carbon atom to which the OH group is attached. Hence, the correct IUPAC name for the given compound will be as follows:
Compound B ⇒4−methylbicyclo[3⋅2⋅0]heptane
The given compound is structurally represented as follows:
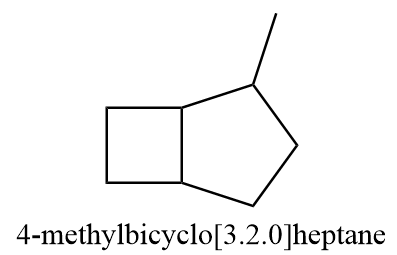
It is a substituted bicyclic compound and according to IUPAC, first all the carbon atoms present on the ring will indicate the name of the parent name of alkane. Secondly, the number of carbon atoms present to the left, right and above bridgehead carbons are denoted in square brackets like [a⋅b⋅c] and the number a, b and c are listed from highest to lowest value.
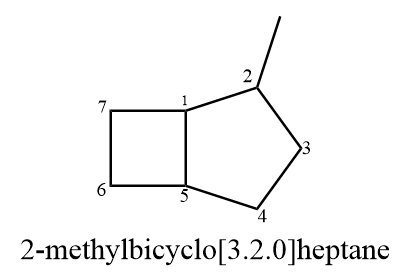
If any substituent groups are present in the compound, then numbering starts from bridged carbon and proceeds in the direction of the longest bridge. The IUPAC name of the given compound will follow the general rule that the numbering is done in such a way that substituent groups get minimum allocation. Hence, the correct IUPAC name for the given compound is as follows:
Compound C ⇒ Butane-1,2-dione
The given compound is structurally represented as follows:
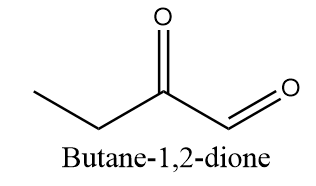
In the compound, two C=O groups are given in which the first C=O group represents aldehyde and the second C=O group represents ketone. According to IUPAC, if both ketone and aldehyde groups are present in the compound, then the aldehyde group is given priority over the ketone group while naming. Hence, the correct IUPAC name for the given compound is as follows:
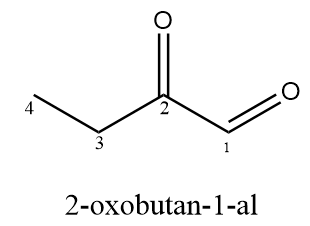
Compound D ⇒ 4-ethyl-5-methylcyclohexene
The given compound is structurally represented as follows:
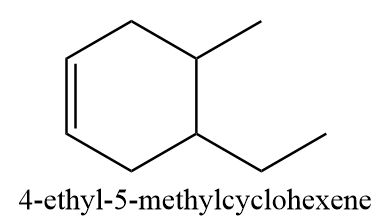
According to IUPAC, if the number of carbon atoms in the alkyl groups attached to the ring are less than the number of carbon atoms in the ring, then the ring is considered as the parent chain. If there is a double bond present in the ring, then the numbering will be done in such a way that the position of the double bond always gets first allocation. The numbering in the given compound is done as follows:
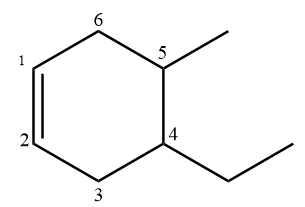
Hence, the given name for this compound is correct according to IUPAC.
Therefore, option (D) is the correct answer.
Note:
It is important to note that if a compound consists of more than one substituent group or functional group, then numbering of chains is done according to priority order but the naming is always done in the alphabetical order irrespective of the value of allocation of substituent group.
