Question
Question: Which among the following is square pyramidal in shape? (A) \( PC{l_3}{F_2} \) (B) \( Xe{O_3}{F...
Which among the following is square pyramidal in shape?
(A) PCl3F2
(B) XeO3F2
(C) IF5
(D) SbF3
Solution
The shape of a molecule can be predicted with the help of hybridization. The square pyramidal shape is made of six equally spaced orbitals which are at an angle of 900 . The orbitals for square pyramidal are sp3d2 . So we will find the hybridization of the molecules and the molecule having the hybridization sp3d2 will have the square pyramidal shape or geometry.
Complete step by step answer
Now we will try to find out the hybridization of molecules to predict their shape or geometry. Hybridization is the concept of merging an atomic orbital from a hybrid orbital. So, let’s consider the molecule IF5 . The hybridization depends on the central metal atom. So, here the central metal atom is Iodine. Now we know that the electronic configuration of Iodine is I to[Kr]4d105s25p5 . Now with the help of electronic configuration, we can find out the total number of valence electrons. So, the total number of valence electrons in Iodine which has one electron less than the stable noble gas electronic configuration is 7 . Now we know that 7 electrons are available for bonding in Iodine. Now we will study the chemical structure of the IF5 molecule. The structure is given below.
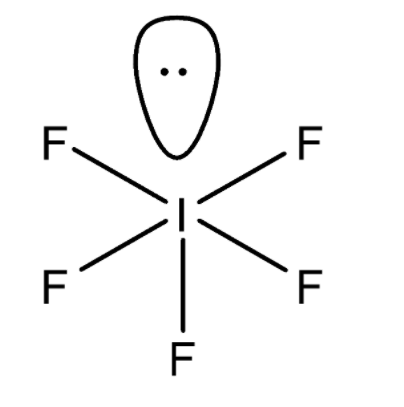
Out of 7 electrons, five electrons are bonded with fluorine atoms and the rest of the 2 electrons form 1 lone pair over Iodine. Now we will calculate the sum of total bonded atoms and the lone pair which is here 6 which means one s , three p , and two d orbitals. Hence, the hybridization is sp3d2 and the shape of the molecule is square pyramidal.
Therefore, the correct option is (C).
Note:
The shape or the geometry of the molecule also depends on the lone pair. The shape of the molecule PCl3F2 , XeO3F2 and SbF3 are Trigonal bipyramidal, Trigonal bipyramidal, and tetrahedral respectively.
