Question
Question: Which among the following is not a planar molecule? A. \({\text{BC}}{{\text{l}}_3}\) B. \({{\tex...
Which among the following is not a planar molecule?
A. BCl3
B. C2H4
C. SF4
D. H2O
Solution
Hybridization is the concept of mixing atomic orbitals into new hybrid orbitals with different energy, shape than the atomic orbitals suitable for pairing of electrons to form chemical bonds.
There are three types of hybridization-sp,sp2,sp3,sp3d,sp3d2,sp3d3
Complete step by step answer:
A. 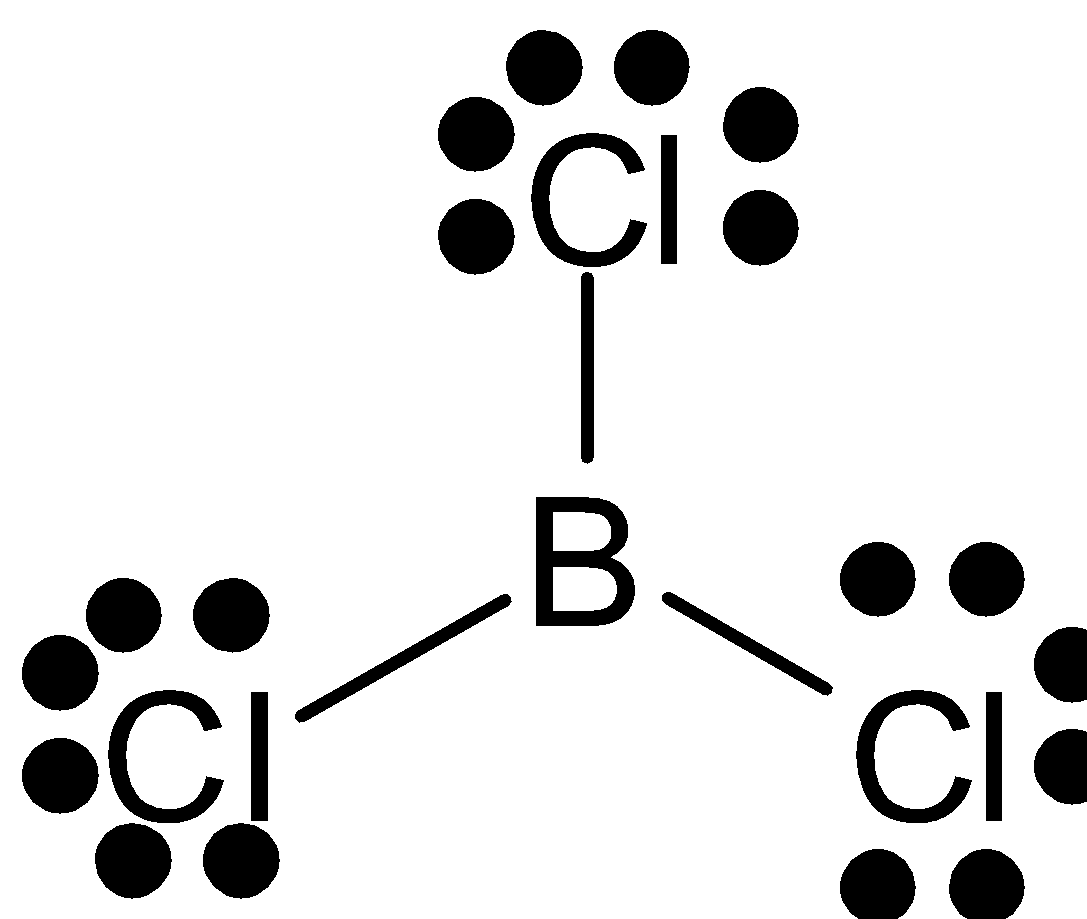
In this compound, the central atom is boron. It is sp2 hybridized. Boron has an electronic configuration 1s22s22p1. For bonding boron with three chlorines, it needs three unpaired electrons. One electron is moved from 2s to 2p. Thus these form three half-filled sp2 hybrid orbitals. This will overlap with the chlorine’s unpaired electron. The bond angle is 120∘ and its geometry is trigonal planar.
B. 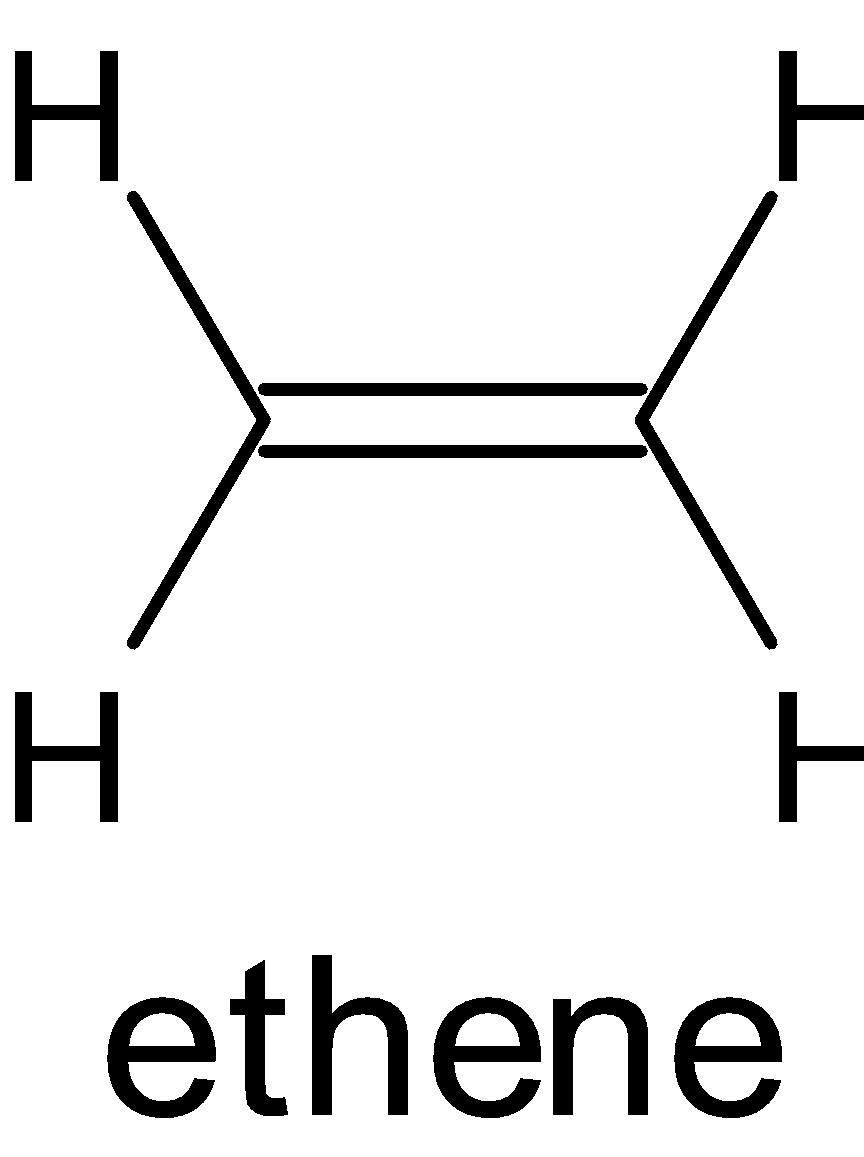
There are two carbons in this molecule and both of them are sp2 hybridized. Each carbon is in trigonal planar geometry. In this compound, carbon-hydrogen has σ and π bonds. π bonds are formed by side to side overlap. The side-to side overlap is between unhybridized p orbital of one carbon to other carbon.
Hence it is planar.
C. 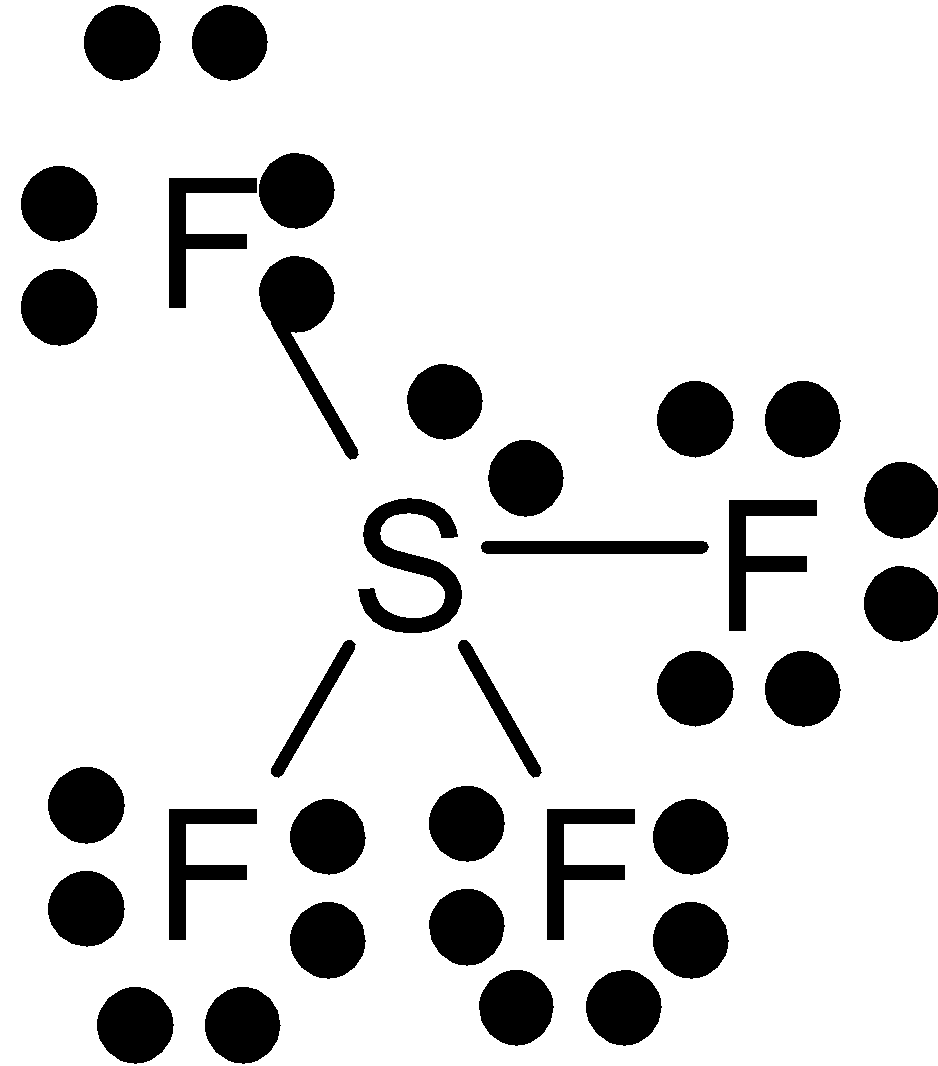
Sulfur has a lone pair of electrons since five of the pair of electrons bonded with fluorine. Its geometry is trigonal bipyramidal. It has two bond angles. The lone pair of electrons are at equatorial position because it needs more space than the bonds. Thus it is not planar.
D. 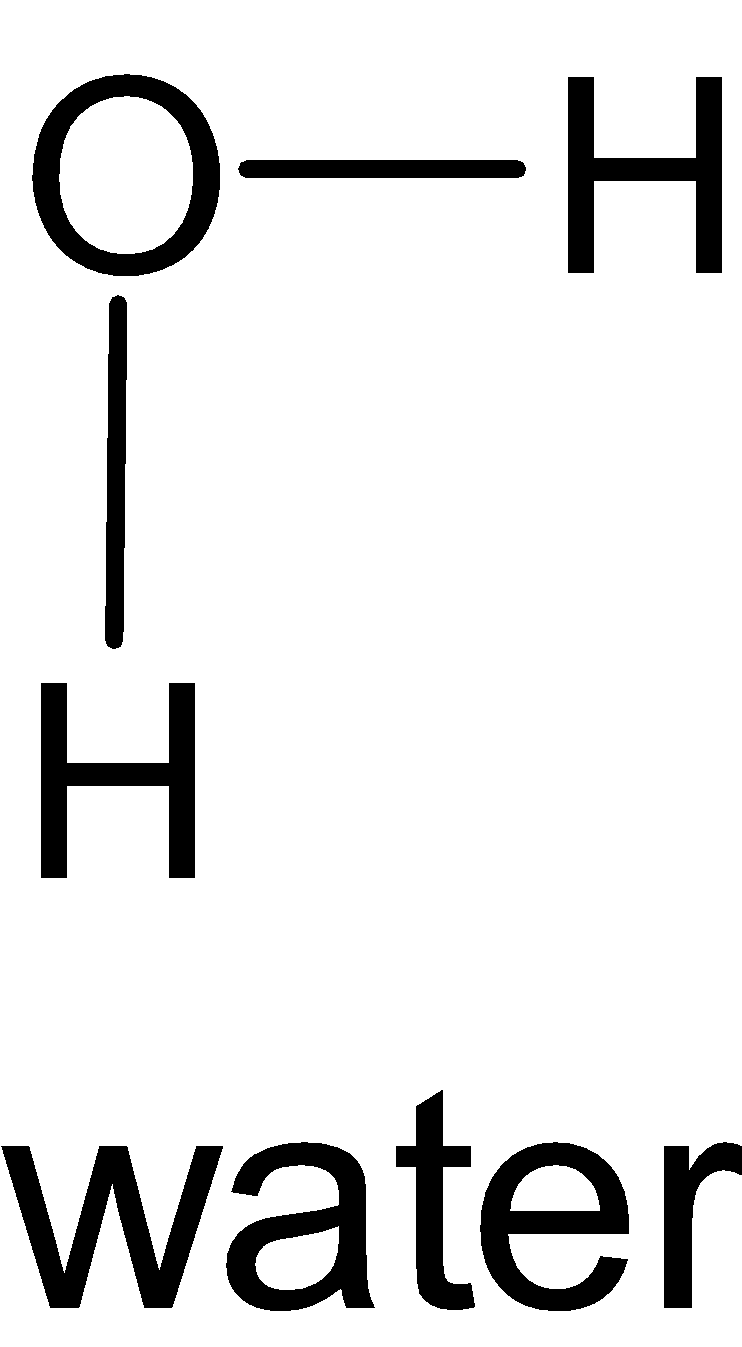
It has a V or bent shape. It lies in a plane.
Hence only SF4 molecule is not planar.
Thus, the correct option is C.
Note:
Valence bond theory is responsible for this concept. It explains about different hybridization and thereby determining the geometry of the molecules. It explains the formation of covalent bonds using quantum mechanics.
