Question
Question: Which among the following free radicals is most stable?...
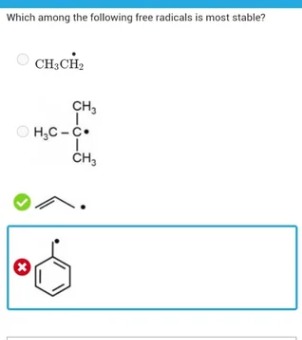
Which among the following free radicals is most stable?
A
CH3CH2·
B
(CH3)3C·
C
CH2=CH–CH2·
D
C6H5–CH2·
Answer
C6H5–CH2·
Explanation
Solution
• Stability of free radicals increases with the extent of electron delocalization (resonance) and hyperconjugation.
• Alkyl radicals: tertiary > secondary > primary due to hyperconjugation.
• Allyl radical (CH2=CH–CH2·) is resonance‑stabilized (2 resonance forms).
• Benzyl radical (C6H5–CH2·) has even greater resonance delocalization into the aromatic ring (4 resonance forms).
• Therefore, benzyl radical is the most stable among the given species.
