Question
Question: Which among the following compounds will give secondary alcohol in reacting with Grignard reagent fo...
Which among the following compounds will give secondary alcohol in reacting with Grignard reagent followed by acid hydrolysis?
I.HCHO
II.C2H5CHO
III.CH3COCH3
IV.RCOOC2H5
The correct option is:
A) Only II
B) Only III
C) II and IV
D) III and IV
Solution
All aldehydes except formaldehyde gives secondary alcohol on reaction with Grignard reagent followed by acid hydrolysis. Ketone and ester on reaction with Grignard reagent followed by acid hydrolysis give tertiary alcohol.
Complete step by step answer:
The given compounds to us are:
HCHO : Formaldehyde
C2H5CHO = Propionaldehyde
CH3COCH3= Acetone
RCOOC2H5= Ester
The reaction of formaldehyde (HCHO) with Grignard reagent followed by acid hydrolysis is as follows:
Formaldehyde on reaction with Grignard reagent followed by acid hydrolysis gives primary alcohol.
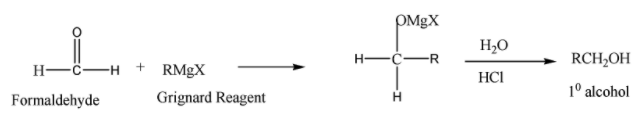
All other aldehydes on reaction with Grignard reagent followed by acid hydrolysis give secondary alcohol.
So, the reaction of propionaldehyde (C2H5CHO) is as follows:
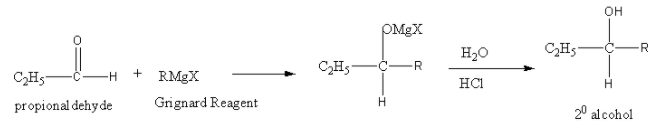
Ketone and ester give tertiary alcohol on reaction with Grignard reagent followed by acid hydrolysis.
So, the reaction of acetone (CH3COCH3) is as follows:
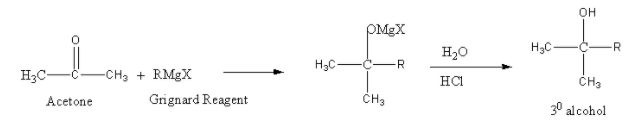
The reaction of ester (RCOOC2H5) with Grignard reagent followed by acid hydrolysis is as follows:
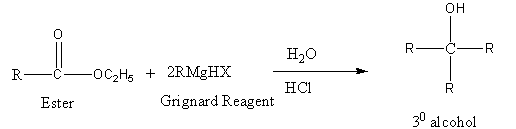
Thus, only Propionaldehyde (C2H5CHO) gives secondary alcohol on reaction with Grignard reagent followed by acid hydrolysis.
Hence, the correct option is (A) only II.
Note: Ketone reacts with 1 equivalent of Grignard reagent while ester reacts with 2 equivalents of a Grignard reagent to give tertiary alcohol. Since formaldehyde does not contain an alkyl group it is the only aldehyde that gives primary alcohol on reaction with the Grignard reagent. While other aldehydes give secondary alcohol.
