Question
Question: Which among the following are peroxo acids of sulphur? [A] \({{H}_{2}}S{{O}_{3}}\) [B] \({{H}_{...
Which among the following are peroxo acids of sulphur?
[A] H2SO3
[B] H2S2O3
[C] H2S2O8
[D] H2SO4
Solution
To answer this, you should know that a peroxo acid is an oxoacid containing a peroxy linkage i.e. an O – O linkage. Draw the structures of the given acids to find the correct answer here. Peroxo acid of sulphur is also known as Marshall’s acid.
Complete Solution :
To answer this question, we have to understand the meaning of peroxo first.
We know that peroxy linkage is an oxygen – oxygen linkage i.e. O – O linkage. Oxo-acids containing these O – O linkage are known as peroxo acids.
Here, we have been asked about peroxo acids of sulphur. Oxo-acids of sulphur with a peroxy linkage are known as peroxo acids of sulphur.
Now, let us go through each of the options to find out the peroxo acids of sulphur among the given options.
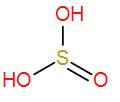
- Firstly we have H2SO3. It is an inorganic compound and is known as sulphurous acid. We have to draw its structure to find out if it has a peroxy linkage.
We can see it has no O – O linkage therefore it is not the correct answer.
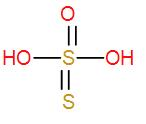
- Then we have H2S2O3. It is also an inorganic acid and is known thiosulphuric acid. It is an oxo-acid of sulphur and we can draw its structure as-
It has no O – O linkage therefore it is not a peroxo acid either.
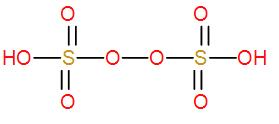
- Next we have H2S2O8. It is an inorganic compound and it is known as peroxydisulfuric acid. It is also known as Marshall’s acid. Here the two sulphur atoms are bonded by a peroxy group. We can draw its structure as-
We can see it has an O – O linkage so this is a peroxo acid of sulphur.
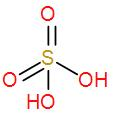
- And lastly we have H2SO4. We know that it is an inorganic acid and is known as sulphuric acid. We can draw its structure as-
It does not have any O – O linkage so it is not a peroxo acid.
We can understand from the above discussion that peroxydisulfuric acid is a peroxo acid of sulphur.
So, the correct answer is “Option C”.
Note: In peroxydisulfuric acid, sulphur is present in its +6 oxidation state. Both +4 and +6 oxidation states of sulphur are common but +6 oxidation state is strongly acidic and thus is a stronger oxidising agent. Peroxydisulfuric acid is industrially used as a strong oxidising agent and it can be prepared by reaction of chlorosulfonic acid with hydrogen peroxide.
2ClSO3H+H2O2→H2S2O8+2HCl
