Question
Question: Which among the following are isostructural? A)\(C{O_2},{I_3}^ - \). B)\(Xe{O_2}{F_{2,}}S{F_4}\)...
Which among the following are isostructural?
A)CO2,I3−.
B)XeO2F2,SF4.
C)SO42−,NO3−.
D)CIF3,XeF2.
Solution
We know that the isostructural species are the compounds that have the same structure. For finding the isostructural species we have to find the hybridization of every species of the central atom.
Complete step by step answer:
Let us see the structure of species in options,
The Lewis structure of CO2 is,
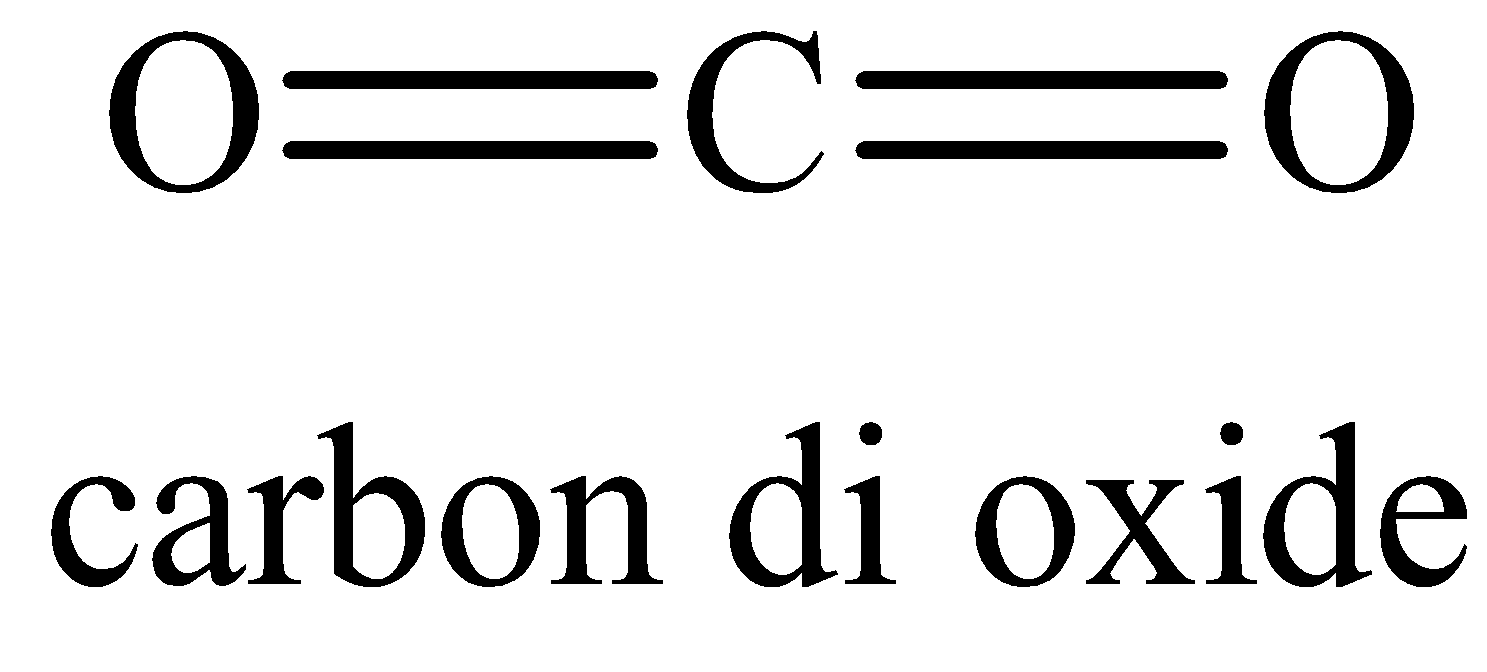
The carbon atom has two electron domains and two of them are in bonding which means that the geometry is linear and it is sp hybridized.
The Lewis structure of I3− is,
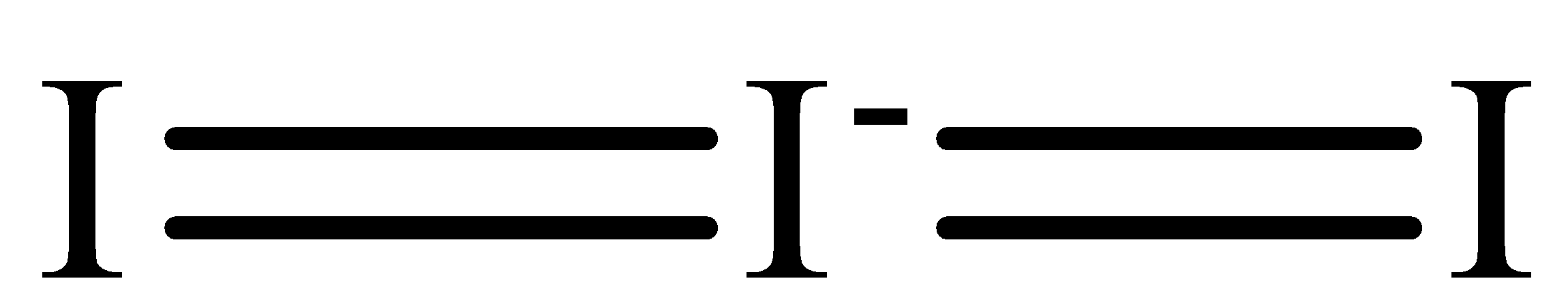
The iodine atom has two electron domains and two of them are in bonding which means that the geometry is linear and it is sp hybridized.
Therefore, the species in option A are isostructural.
The structure of XeO2F2 is,
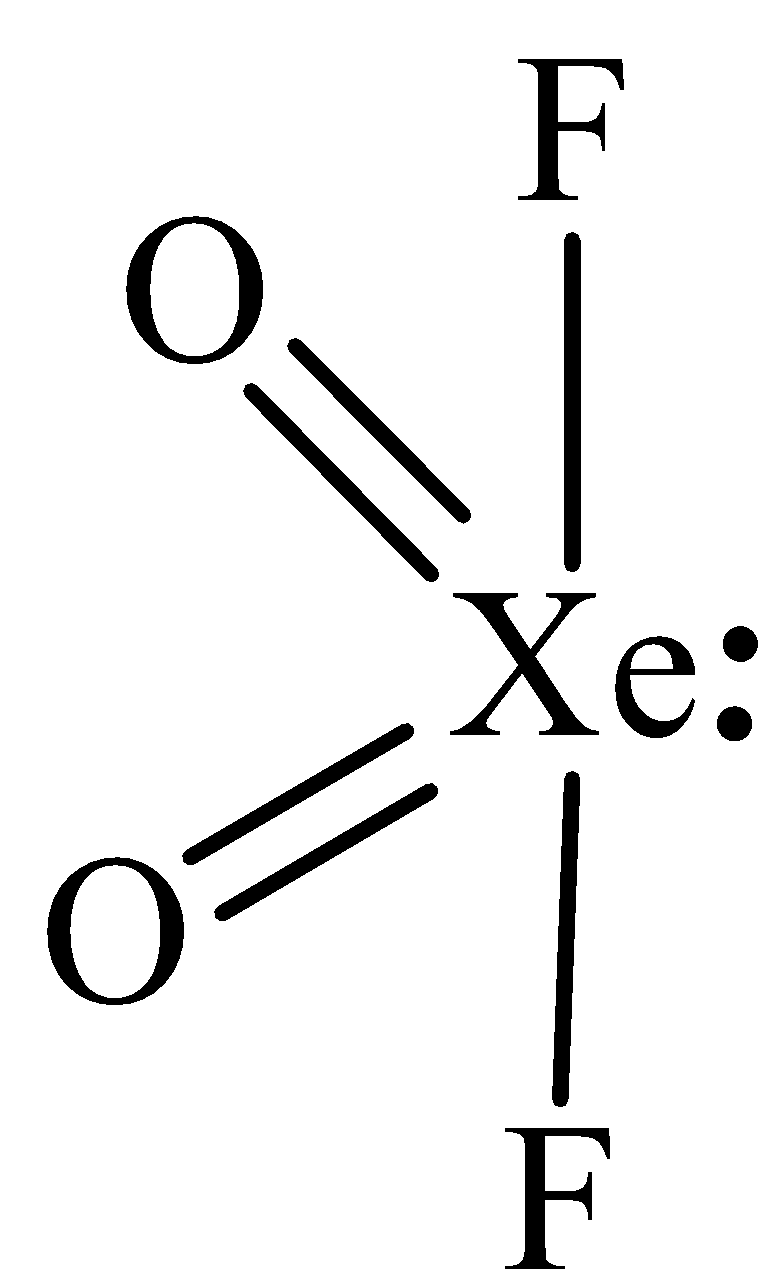
In XeO2F2, the central atom is xenon has one lone pair of electron and four bonding domains. The hybridization of XeOF4 is sp3d and the molecular geometry is see saw.
The structure of SF4 is,
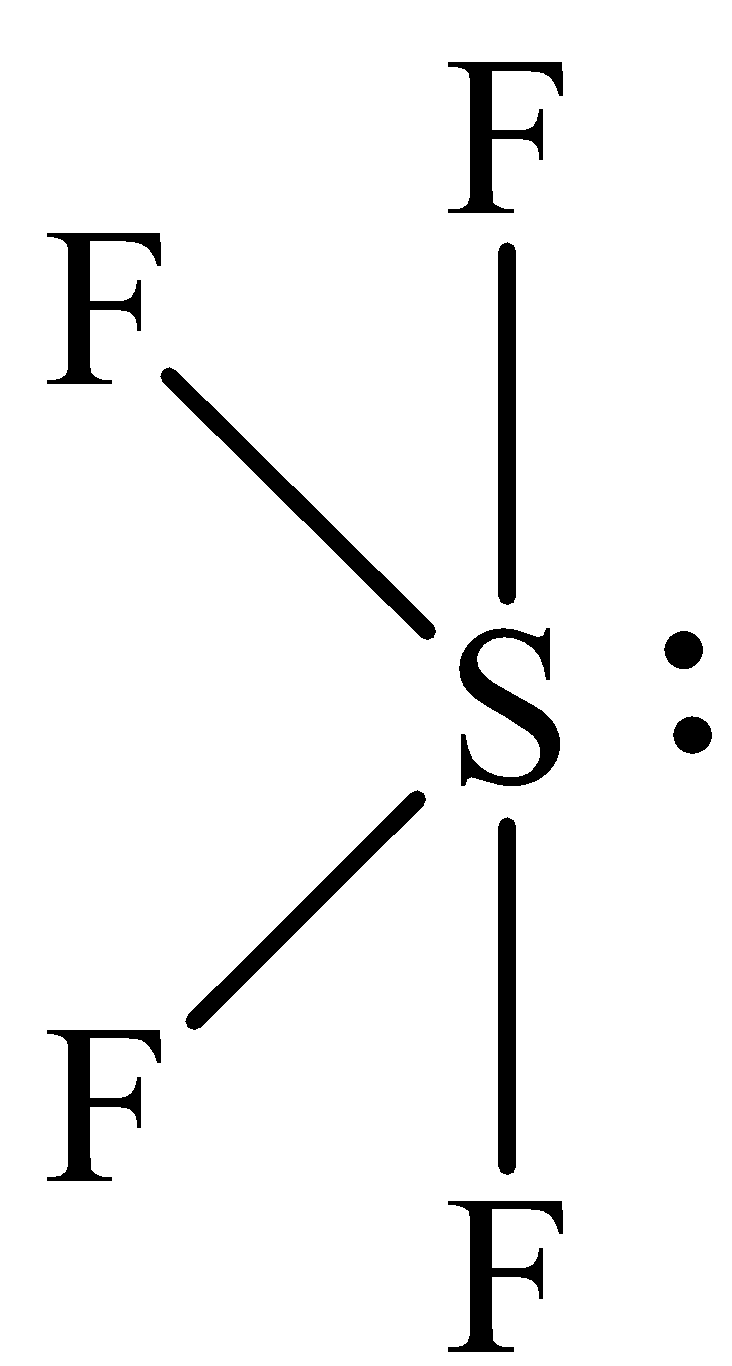
The SF4 have five electron domains and four of them are in bonding which means that the geometry of SF4 is see-saw. The see-saw geometry around the sulphur means that it has sp3d hybrid orbitals on sulfur atom.
Therefore, the species in option B are isostructural.
The structure of NO3 - is,
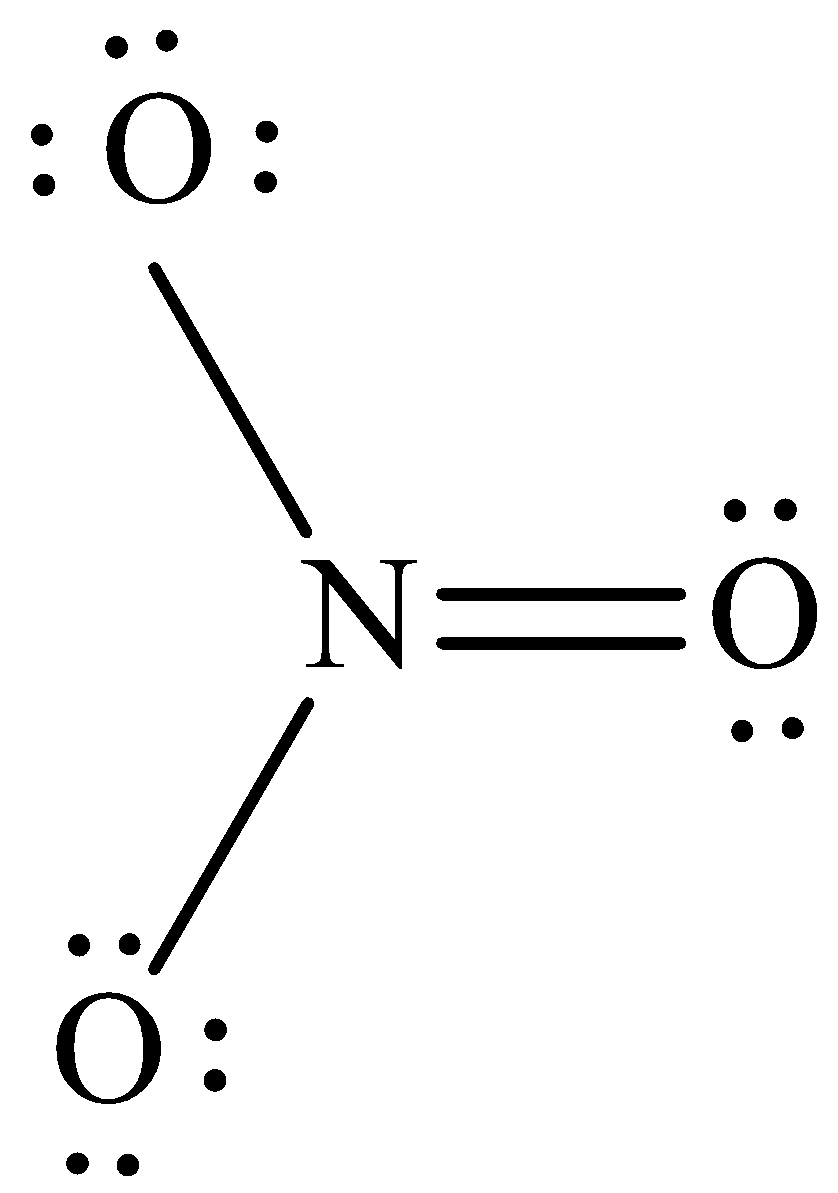
The NO3 - have three electron domains and all of them are in bonding which means that the geometry of NO3 - is trigonal planar. The trigonal geometry around the N means that it has sp2hybrid orbitals on N atom. Thus the orbital which forms sigma bonds is sp2 hybrid orbital.
The Lewis structure of SO42− is,
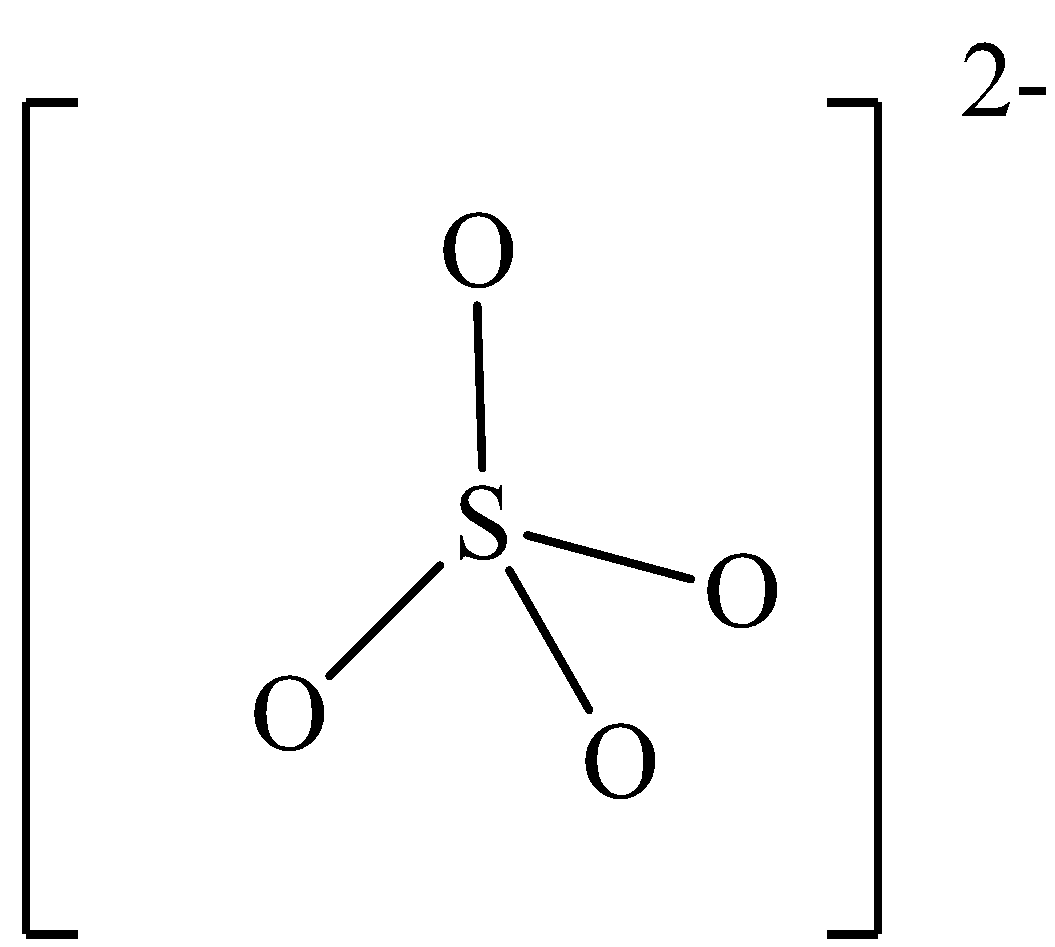
The central sulphur atom has 4 electron domains and are of them are in bonding which means that the carbon is sp3 hybridized and the bond angle of carbon in methane thiol is 109.5o.
Therefore, the option C is incorrect.
The Lewis structure of xenon difluoride is,
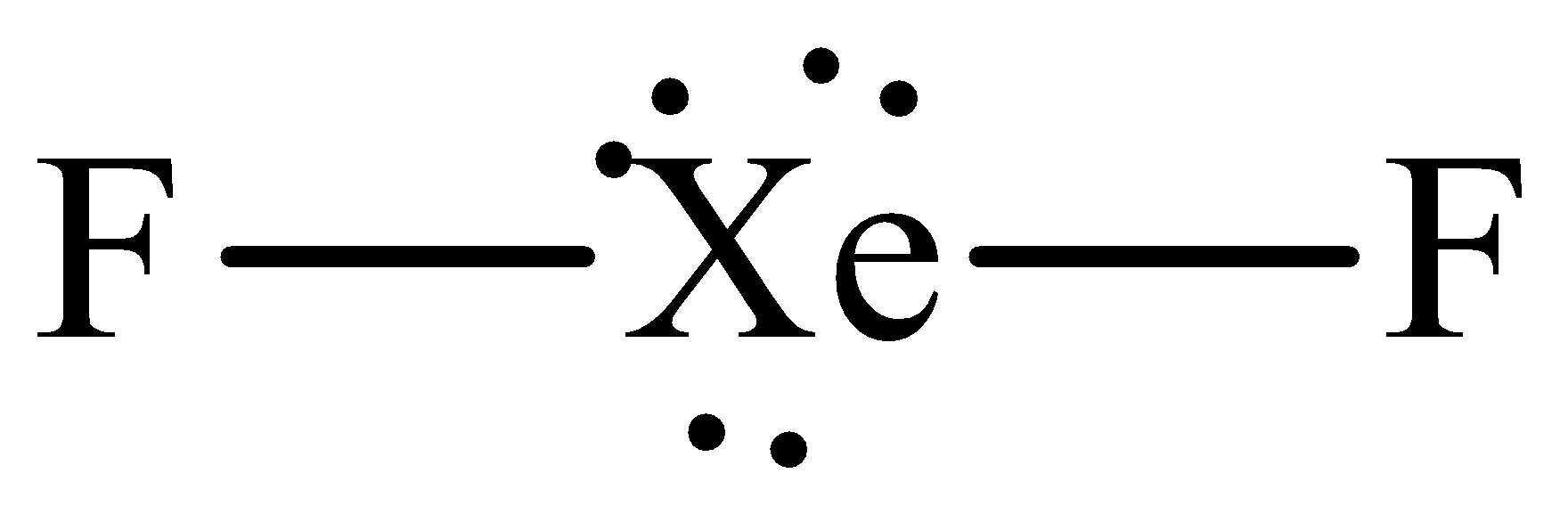
The steric number of central xenon atom is five which means that it is sp3d hybridized but it has only two bonding electrons thus it adopts the linear geometry with the bond angle of 180o.
The structure of chlorine trifluoride is,
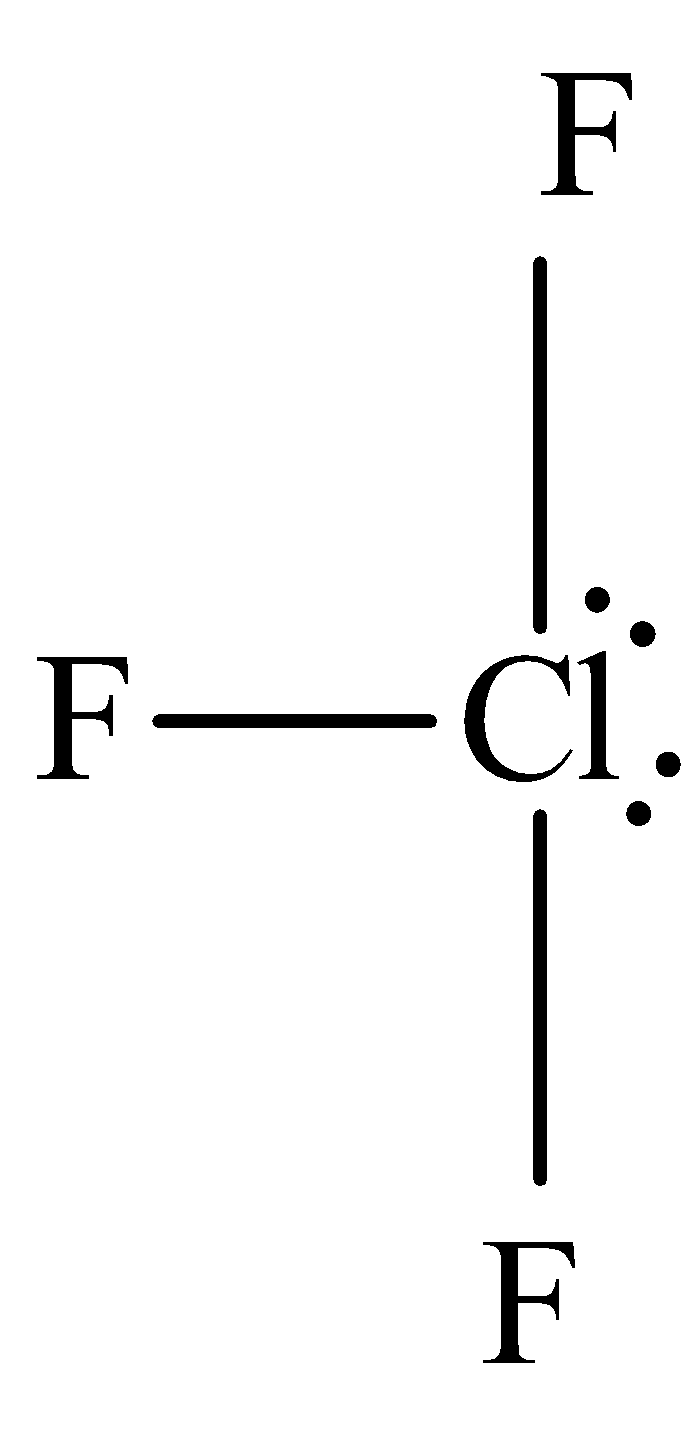
Chlorine trifluoride is sp3d hybridized and the molecular geometry is T-shape. Hence xenon trifluoride and chlorine trifluoride are not structural molecules.
Therefore the option D is incorrect.
Hence, all the given options (A) and (B) are correct.
Note:
We also remember that those species that have the same number of electrons are called isoelectronic species.
Example:
The total number of electrons in CN - is ten.
The total number of electrons in N2 is ten.
The species CN - ,N2 are isoelectronic with each other.
