Question
Question: Which amine is most basic. A. Proline B. Serine C. Lysine D. Alanine...
Which amine is most basic.
A. Proline
B. Serine
C. Lysine
D. Alanine
Solution
The basicity of a compound is going to depend on the capability of donating electrons to others. The strength of the basicity of the atoms or molecules is going to decide by the donating capability of electrons.
Complete step-by-step answer: - In the question it is asked to find the more basic compound among the given options.
- In the options they have given the amino acids are the molecules.
- To know about the basicity of the given amino acid we should know the structure of them.
- The structure of proline is as follows:
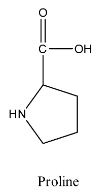
- The structure of serine is as follows:
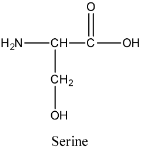
- The structure of lysine is as follows:
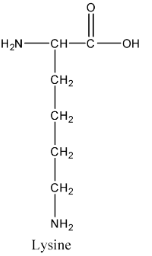
- The structure of alanine is as follows.
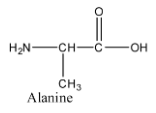
- Out of the four compounds given in the options Lysine is the amino acid two amino groups present int.
- We know that amine groups are going to act as a good electron donor.
- Then amine groups are basic in nature.
- Since the lysine amino acid contains two amine groups it is going to act as a good base when compared to the remaining three amino acids given in the options.
Note: All amino acids are not going to act as good bases, the amino acids which contain more number of amine groups are only going to act as a good base. Lysine and aspartame are going to act as good bases.
