Question
Question: Which alkene gives only acetone on ozonolysis?...
Which alkene gives only acetone on ozonolysis?
Solution
Ozonolysis is defined as a reaction in which unsaturated bonds of alkenes, alkynes or azo compounds are broken down with ozone. Alkenes and alkynes form organic compounds in which the multiple carbon – carbon bond is replaced by a carbonyl group.
Complete answer:
To solve this question, let's first understand the mechanism of ozonolysis of alkenes. When alkenes undergo ozonolysis in the presence of zinc and water, the following reaction takes place:
….(i)
Here, R, R′, , represent the chains of carbon.
The double bonds are broken down by ozone as shown below:
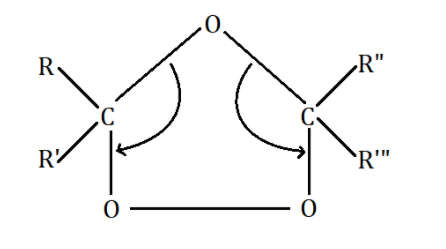
As we can see, the oxygen atom forms bonds with the two carbon atoms where double bonds resided earlier to create an intermediate called ‘Criegee intermediate’. This intermediate decomposes to give us the required carbonyl group i.e., ketones.
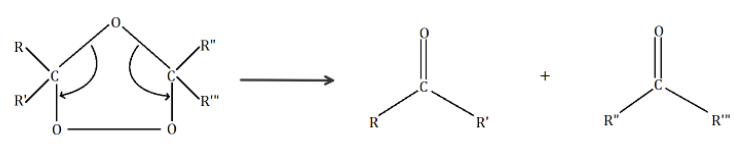
In the given question, we have to identify an alkene compound which on ozonolysis produces acetone only. We know that the formula of acetone is (CH3)2C=O or CH3C(O)CH3.
If we look in the reaction (i), we have to use a symmetrical alkene to get acetone. Hence, in reaction (i), we only have to replace R, R′, , with CH3. On replacing we get CH3C(CH3)=C(CH3)CH3 which is 2,3− Dimethyl −2− butene. It is a symmetrical alkene.
CH3C(CH3)=C(CH3)CH3+O3Zn/H2O2(CH3)2C=O
Hence, 2,3− Dimethyl −2− butene is the alkene that gives only acetone on ozonolysis.
Note:
Ozonolysis is also used to produce aldehydes. To create aldehydes, alkene compounds with formula R−CH=CH2 are used to produce the aldehydes R−CHO and HCHO. Here, R represents the carbon chain.
In certain reactions of ozonolysis, aldehydes and ketones are both produced. These reactions use unsaturated compounds having formula to give ketones R−C(O)−R′ and aldehydes .
Ozonolysis is considered as an organic redox reaction.
