Question
Question: which alkane would have only primary and tertiary carbon? (A) Pentene (B) \( 2 - \) methyl butan...
which alkane would have only primary and tertiary carbon?
(A) Pentene
(B) 2− methyl butane
(C) 2,2− dimethylpropane
(D) 2,3− dimethyl butane
Solution
Primary carbons are carbons attached to one other carbon. Secondary carbons are attached to two other carbons. Tertiary carbons are attached to three other carbons. Quaternary carbons are attached to four other carbons. We can’t go higher than that. To have five substituents, we’d need 10 electrons around carbon, a clear violation of the octet rule.
Complete answer:
A primary carbon is a carbon atom which is bound to only one other carbon atom. It is thus at the end of a carbon chain. In the case of an alkane, three hydrogen atoms are bound to a primary carbon. A tertiary carbon atom is a carbon atom bound to three other carbon atoms. For this reason, tertiary carbon atoms are found only in hydrocarbons containing at least four carbon atoms. Tertiary carbon atoms can occur, for example, in branched alkanes, but not in linear alkanes.
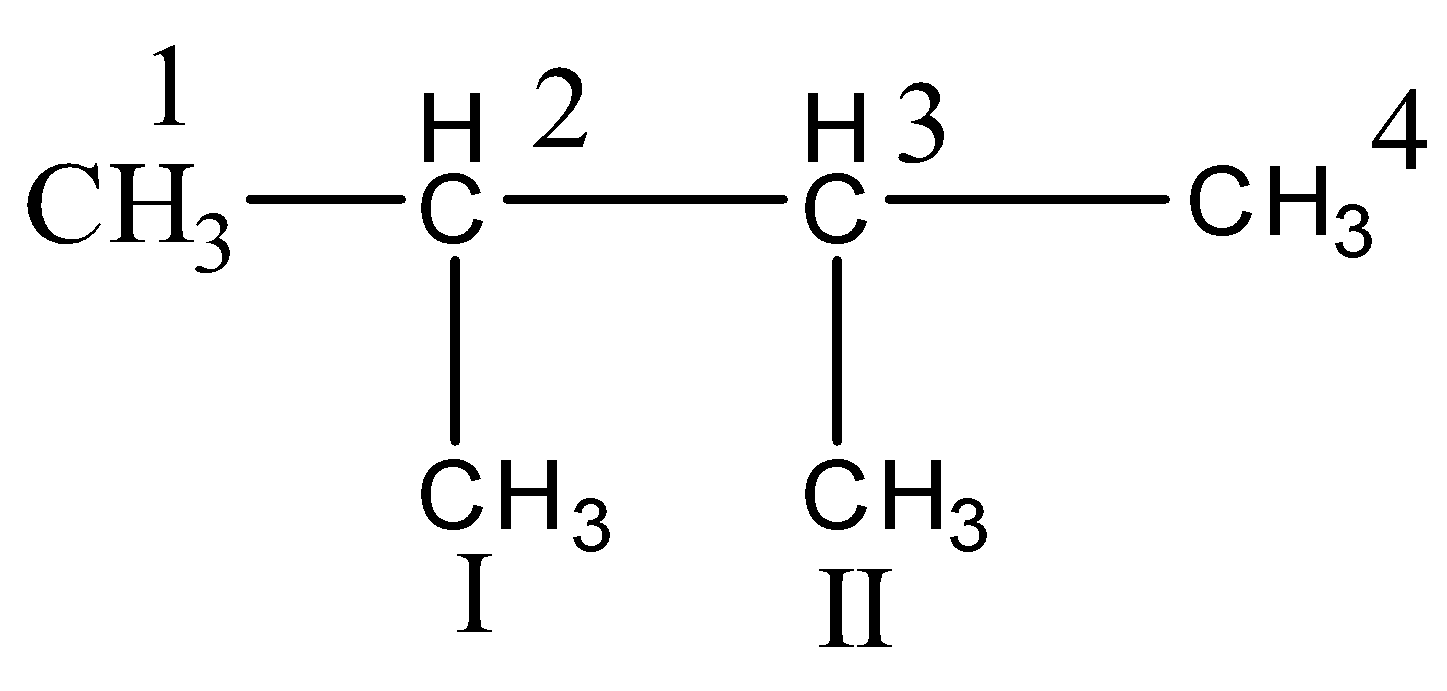
In 2,3− dimethyl butane, we have
C1,C2,CI,CII are primary carbons
C2,C3 are tertiary carbons.
Hence, we have only the primary and tertiary carbon.
So, the correct answer is (D) 2,3− dimethyl butane.
Note:
We use the same terminology for carbocation. A primary carbocation is attached to one other carbon, a secondary to two, and a tertiary to three. We can’t have a quaternary carbocation without violating the octet rule either. Alcohols also follow the primary/secondary/tertiary nomenclature. The rule for alcohols is that they are named according to the number of carbons attached to the carbon bearing the hydroxyl group.
