Question
Question: When \(\text{N}{{\text{H}}_{\text{3}}}\) is treated with HCl, \(\text{H -- N -- H}\) bond angle: ...
When NH3 is treated with HCl, H – N – H bond angle:
A. increases
B. decreases
C. remains same
D. depends upon temperature
Solution
Hint: The increase or decrease of the bond angle of a compound is determined by the s character. By s character we mean the s hybridisation. So, to determine the increase or decrease of the bond angle, we need to find the hybridisation of the compound.
Complete step-by-step answer:
We know that NH3 has a bond angle of 107 !!∘!! . This bond angle is less as compared to the tetrahedral angle, owing to the increase in the repulsion to bond pairs. This happens due to the presence of lone pairs of nitrogen.
Now on reacting with HCl, NH3 results in the formation of NH4Cl. The reaction is given below:
NH3 + HCl NH4Cl
In this case, NH4+ has no longer any lone pair at nitrogen. Hence, the ammonium ion is perfectly tetrahedral, just like the CH4. So, now the bond angle is 109.5 !!∘!! .
Therefore, we can say that when NH3 is treated with HCl, H – N – H the bond angle increases.
So, the correct answer is Option A.
Both NH3 and NH4+ are sp3 hybridized. Here is the structure of both of the structures.
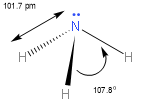
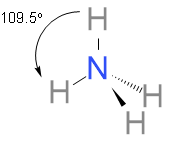
Note: Angle between two adjacent bonds at an atom in a molecule made up of three or more atoms is known as the bond angle. Bond angle is affected by the presence of a lone pair of electrons at the central atom.
The electronegativity of elements decreases as we move down the group. The lone pair - bond pair repulsion is stronger as compared to the lone pair - lone pair or bond pair - bond pair interaction. But as electronegativity decreases, the repulsion also decreases which results in a decrease in bond angle.
