Question
Question: When sulphur monochloride is saturated with chlorine, the compound formed is A) \(SC{{l}_{6}}\) ...
When sulphur monochloride is saturated with chlorine, the compound formed is
A) SCl6
B) SCl4
C) SCl2
D) S2Cl2
Solution
Sulphur has 2 free electrons in the outermost subshell to get covalent bonding in ground state and in an excited state it has 6 electrons free in its last shell. So, in less or saturated amount of chlorine sulphur be in ground state.
Complete step by step solution:
Now formula of sulphur monochloride is S2Cl2
And electronic configuration of Sin ground state be like that shown in diagram
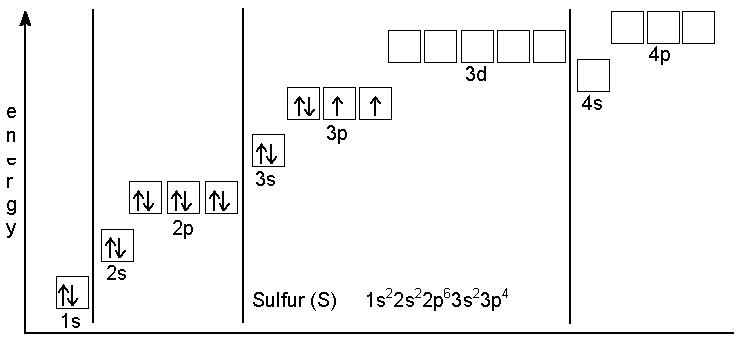
There are two atoms in last 3p subshell when sulphur is in ground state so sulphur breaks from its S−S Bond in S2Cl2 and make a coordinate bond with Cl in which there is a last vacant shell in outer subshell.
Electronic configuration of chlorine is [Ne] 3s2 3p5
So, the reaction be like-
S2Cl2+Cl2→SCl2
Then in the excess or in saturated quantity of chlorine gas it gives sulphur dioxide as a product.
Hence Option (C) is correct.
Additional Information:
Disulfur dichloride is the inorganic compound of sulfur and chlorine with the formula S2Cl2 Some alternative names for this compound are sulfur monochloride, disulphur dichloride and sulphur monochloride.Its structure be like:
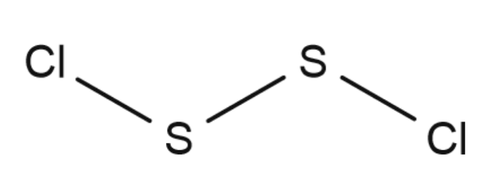
There is a covalent bond between sulphur and sulphur to complete inertness of its outer shell.
It is produced by partial chlorination of elemental sulfur. The reaction proceeds at usable rates at room temperature. In the laboratory, chlorine gas is led into a flask containing elemental sulfur. As disulfur dichloride is formed, the contents become a golden yellow liquid:
S8 + 4 Cl2 → 4 S2Cl2, ΔH = −58.2 kJ/mol
Note: It gives different reactions when chlorine is not present in sufficient amounts. So the saturated word in question decides many factors in which direction the reaction goes. It also depends on the stability of the final product. Also, temperature during reaction also plays a major factor on the final product.
