Question
Question: When sodium lauryl sulphate is dissolved in water in an appreciable amount, micelle formation takes ...
When sodium lauryl sulphate is dissolved in water in an appreciable amount, micelle formation takes place. Which of the following options is correct regarding the formation process?
(A) Micelle formation can occur at any temperature.
(B) At substantially lower concentrations as concentration increases, conductivity should decrease.
(C) On dilution micelle molar conductance should decrease.
(D) The micelle formation in a given case will lead to formation in given cases will lead to formation of positively charged colloids.
Solution
We will learn about what micelle and then about the conditions of micelle formation as to at what temperature or concentration micelle formation takes place. And a micelle is an electrically charged particle formed by an aggregate of molecules.
Complete step by step answer:
According to colloidal chemistry micelles are the aggregate of the surfactants molecule which are dispersed in liquid to form a colloid solution. When a micelle is formed in water it typically has two characteristic structure differentiation. One part of the micelle is hydrophilic (head region) which is in contact with the water molecules and other part is hydrophobic (tail region) which is water repellent and is aggregated in the center. This forms a bilayer in the solution.
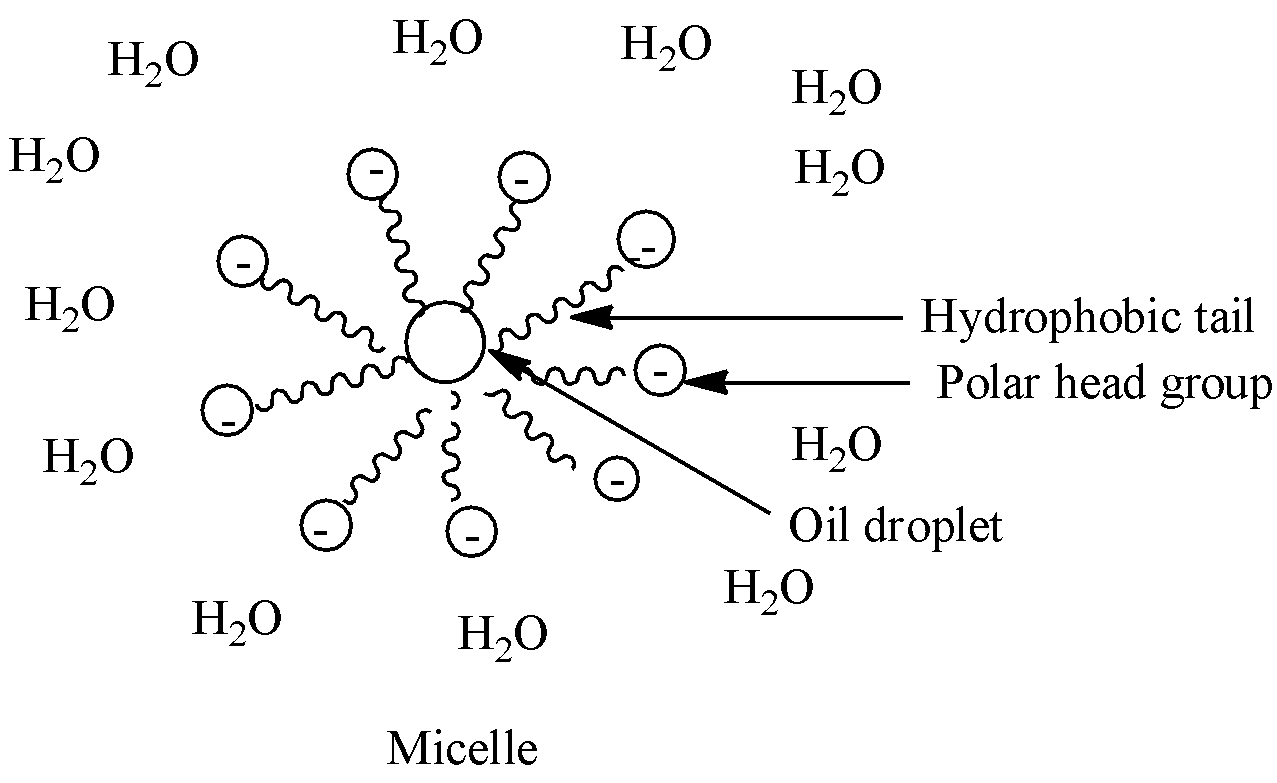
The head part here is hydrophilic and forms the outer layer of micelle aggregate and the tail part is hydrophobic and forms the inner layer.
Micelle formation can only occur above the critical micelle concentration and the temperature should be greater than critical temperature or krafft temperature.
When we increase the concentration, the conductivity increases appreciably.
Anionic surfactant produces negative charged colloid and cationic surfactant produces positively charged colloid.
So, the correct answer is Option C.
Additional information:
The most useful application of micelle formation is working soap. Due to formation of bilayer in micelles which are hydrophobic and hydrophilic the dirt can be easily taken out. The dirt is mostly fats and organic compounds which attach to hydrophobic ends and after washing the hydrophilic ends make them go out from clothes.
Note: Micelles have electrostatic attraction between their ions that surround them in solution. Ionic micelle changes many properties of the mixture for example electrical conductivity etc. Adding compounds in solution which can weaken the electrostatic force produce large micelles.
